Abstract
The fauna of streams in the High Arctic, dominated by chironomids, is shaped by extreme environmental conditions that represent the physiological limits for benthic invertebrates. Despite their ecological importance, little is known of chironomid life histories, development strategies and the key abiotic drivers limiting larval growth in High Arctic streams. We investigated the larval development and growth in three High Arctic rivers with contrasting water sources, thermal regimes and nutrient characteristics. Populations of the larvae of Diamesa bohemani (Goetghebuer 1932) and Diamesa aberrata (Lundbeck 1898) from two sampling occasions in July and August 2016 were morphometrically analysed to determine life history patterns and instream productivity. Water temperature differences lead to diverging development patterns on local spatial scales. The lowest larval growth was in a groundwater/snowmelt fed stream with low food concentration and quality, suggesting that stream productivity is not primarily water source dependant, but is dependent on the nutrient supply. Glacially influenced streams are clearly more productive than previously assumed, resulting in comparable secondary production to groundwater/snowmelt-fed streams.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streams in the High Arctic are situated in one of the most extreme climatic regions (e.g. Blaen et al. 2014). In addition to the harsh conditions, like year-round low temperatures, a very short snow- and ice-free season and often limited nutrients, climate change effects are projected to alter freshwater ecosystems in the Arctic more drastically than anywhere else in the world (Lods-Crozet et al. 2001, 2007; Prowse et al. 2006; Wrona et al. 2006; Danks 2007; Vincent et al. 2011; Blaen et al. 2013). Like in alpine regions, which are limited by similar environmental conditions (e.g. see Brittain and Milner 2001; Füreder 2007), rivers in the High Arctic are often influenced by a draining glacier in the catchment, leading to highly fluctuating discharges, high turbidity and low channel stability (Docherty et al. 2018a; Füreder and Niedrist 2020). Besides these kryal freshwaters (see Steffan 1971; Füreder 2007) spring or snow-fed streams are comparatively benign ecosystems hypothesized to allow for higher benthic diversity and individual densities due to stable runoff conditions, little sediment load and increased algal growth (e.g. Brown et al. 2003; Lods-Crozet et al. 2007; Blaen et al. 2014). Nevertheless, both stream types in the High Arctic are characterized by only a few months per year of flow and above zero water temperatures, thus representing the physiological limit of habitable aquatic ecosystems for benthic invertebrates (Danks et al. 1994; Lencioni 2004; Lods-Crozet et al. 2007).
Only few, highly adapted organism groups inhabit these exceptionally harsh aquatic ecosystems (Hodkinson et al. 1996; Brittain and Milner 2001; Friberg et al. 2001). Biodiversity of High Arctic streams is low (Lods-Crozet et al. 2001; Blaen et al. 2014; Docherty et al. 2018b), especially on the island archipelago of Svalbard (Coulson et al. 2014) where no Ephemeroptera, Plecoptera or Trichoptera are found in running waters. One of the most successful insect families, dominating the benthic fauna of high altitudinal and latitudinal streams, are the Chironomidae (Diptera), mainly from the genus Diamesa (Milner and Petts 1994; Brittain and Milner 2001; Friberg et al. 2001; Füreder 2007; Lods-Crozet et al. 2007; Schütz and Füreder 2018; Stur and Ekrem 2020). Larvae of cold-stenotherm Diamesa species can locally reach high densities and thereby significantly contribute to the otherwise short food chains of such harsh ecosystems (see Robinson et al. 2014; Niedrist and Füreder 2017 for alpine streams). Despite their small body size, chironomid larvae play an essential role in the self-purification of the water bodies, feeding on autochthonous and allochthonous organic matter and simultaneously representing a key food source for fish (e.g. Svenning et al. 2007; Niedrist and Füreder 2017). Once emerged, the aerial adults are an important food item for birds and their chicks in Arctic regions (Hodkinson et al. 1996). Hence, chironomids are an essential link in the aquatic and terrestric food chain of High Arctic ecosystems.
Despite their abundance and environmental significance, little is known of the ecology of High Arctic chironomid larvae and key environmental drivers. Nevertheless, water source dynamics have been shown to be of importance in structuring lotic chironomid communities in the High Arctic (Lods-Crozet et al. 2007; Blaen et al. 2014). In addition, recent studies from the European Alps (Niedrist and Füreder 2018; Schütz and Füreder 2018) have increased our understanding of chironomid survival strategies. Schütz and Füreder (2018) documented life cycle dynamics and growth patterns of Diamesa (Chironomidae) larvae in a highly glaciated alpine stream and found unexpectedly fast life cycles. They also reported significantly decreasing larval size with decreasing nutrient availability. Despite the very low water temperatures, the chironomid larvae in their sampled stream are obviously able to metabolize a nutritional surplus and hence grow significantly larger in their aquatic life stages. Recently, Füreder and Niedrist (2020) studied chironomid larval size along a natural harshness gradient of decreasing glacial cover in the river catchments but rising water temperatures and found that increased benthic larval size may be a result of such environmental harshness, leading to a competitive advantage for large growing chironomid larvae in cold, highly glaciated streams.
Water temperature is also known to affect life history patterns of benthic invertebrates like egg and larval development time, timing of adult emergence and larval growth rates (e.g. Reynolds and Benke 2005; Sand and Brittain 2009; Schütz and Füreder 2018, 2019). In one of the few studies of cold-stream chironomids, Schütz and Füreder (2018) followed chironomid larval development in a highly glaciated alpine stream along a narrow temperature gradient and found that larval life history was hardly affected by temperature. Other studies, involving greater temperature differences in cold lowland streams report considerable thermal influence on chironomid larval development (e.g. Nolte and Hoffmann 1992), with increasing temperatures considerably accelerating chironomids larval development, leading to early emergence. Comparable effects can be expected in High Arctic streams, but are still uncharted.
In this study we studied the influence of water temperature and nutrient availability on the life history and larval growth of key chironomid species in the High Arctic. Chironomid communities and key environmental components of two spring-fed and one glacier-fed stream on Svalbard were sampled twice a year to follow the larval development. We hypothesise that: (1) larval development is temperature and hence stream type dependent, leading to contrasting larval life strategies between different streams; (2) not only food quantity but also quality limits chironomid larval growth.
Materials and methods
Sampling sites
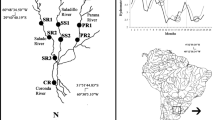
Sampling took place on Spitzbergen, the largest island of the Svalbard archipelago, close to Ny-Ålesund in July 2016 and August 2016. Three streams with markedly different abiotic conditions were investigated on both occasions. The streams were: Bayelva (Fig. 1; sampled 23 July 2016 and 24 August 2016), a glacier-fed (kryal, according to Steffan 1971) river close to Ny-Ålesund; Londonelva (Fig. 1; sampled on 22 July 2016 and 24 August 2016); a spring-fed (rhithral, according to Steffan 1971) stream on the island Blomstrandhalvoya and Stuphalletelva (Fig. 1; sampled on 26 July 2016 and 23 August 2016), a second rhithral stream draining from the Stuphallet bird cliffs, north west of Ny-Ålesund.
Map of the sampling area close to Ny-Ålesund, on Svalbard`s largest island Spitzbergen with the three investigated streams: the glacier-fed Bayelva, spring-fed Londonelva and spring-fed Stuphalletelva. Arrows indicate the approximate location of the sampling site at each stream. Main mountain ranges shown as shaded grey or black areas glaciers as horizontally dashed areas and the location of Stuphallet bird cliffs as a diagonally dashed area
Abiotic conditions
To characterise the key abiotic conditions on macroinvertebrate growth and life cycle patterns in the sampled streams, water temperature and nutrient supply were measured by the following methods. In each stream and on both sampling occasions the upper surface of three permanently wetted, flat stones (grain size 6.3 to 20 cm, as this grain size was dominant in each sampled stream) from the river bottom was brushed off, dissolved in 100 ml water and filtered in equal parts through two glassfibre filters (MN GF-3) to estimate the amount of chlorophyll a and organic material in order to estimate food availability for benthic invertebrates. The six rocks at each stream (three per sampling trip) were chosen randomly. The filters for the estimation of organic material were muffled (2 h, 450 °C) and weighed before filtration. After filtration, these filters were dried (24 h, 60 °C), weighed, burned (2 h, 450 °C) and weighed again. Chlorophyll a on the second filter was analysed according to Lorenzen (1967). The brushed area of the stones was measured to obtain surface area. As Fuller et al. (2004) showed that chlorophyll a concentration of algae can influence benthic invertebrate densities in streams, we divided the amount of chlorophyll a (µg cm−2) by the amount of organic material (µg/cm2) on the brushed stones as measure of food quality, whereby a higher value indicates a higher amount of chlorophyll a and thus higher food quality. For stream characterisation, Mann–Whitney-U tests (IBM SPSS 23, test considered significant with p < 0.05) compared the organic material on the substrate and its quality as potential food for benthic invertebrates. The two sampling trips and three replicates on each trip were treated as independent replicates in the tests. Data were not normally distributed. Besides the statistics, we also calculated means for organic matter and chlorophyll a on the stream bottom (OMStones; ChlaStones) and the food quality for each stream over the two sampling periods to allow for easier stream comparisons. Water temperature of the three investigated streams was tracked with dataloggers (Tidbit v2 Temp Logger, Onset) at 30-min intervals. Mean seasonal water temperature was calculated as the average of all mean daily temperatures between the two sampling trips (40 days, 22 July 2016 to 24 August 2016).
Biotic sampling and species selection
To follow chironomid life cycles and conclude on the larval growth, we sampled benthic larvae in the three streams in July 2016 and August 2016. On each sampling occasion, three replicate, semi-quantitative Surber Samples (0.084m2, 100 µm mesh net) were taken from permanently wetted stream sections reflecting the typical grain size (micro-/mesolithal at Londonelva and micro-/meso-/macrolithal at Bayelva and Stuphalletelva, whereby mesolithal was the dominating grain size in all streams) and habitat conditions. The samples containing the benthic invertebrates were immediately preserved in 75% ethanol. Furthermore, adult chironomids were caught with a sweep net or by hand when sitting on the stream banks. These were also preserved in 75% ethanol. In the laboratory, all benthic invertebrates were handpicked from the Surber samples and the chironomid larvae determined to species following available keys (Wiederholm 1983; Ferrarese and Rossaro 1981; Schmid 1993; Janecek 1998; Makarchenko and Makarchenko 1999; Rossaro and Lencioni 2015a, b; Stur and Ekrem 2020). Chironomid pupae and adult chironomid males were also identified to species (keys: Serra-Tosio 1971; Wiederholm 1989; Langton 1991; Langton and Pinder 2007) to support larval species identification.
In all three study streams near Ny-Ålesund ≥ 80% of benthic numbers were Chironomidae (Füreder and Brittain, unpublished data). In Stuphalletelva Oligochaeta accounted for about 18% of benthos numbers, but much less in the two other rivers (Füreder and Brittain, unpublished data). Among the Chironomidae, Diamesinae were almost totally dominant in the glacier-fed Bayelva, whereas in Londonelva and Stuphalletelva, Orthocladinae were well represented (Füreder and Brittain, unpublished data). Diamesa bohemani (Goetghebuer 1932) and Diamesa aberrata (Lundbeck 1898), two species of the chironomid subfamily Diamesinae, consistently occurred in all three sampled streams. Therefore, larvae of these two species were used for the comparative productivity and development analyses. Lods-Crozet et al. (2007) also recorded these two Diamesa in Bayelva and Londonelva, indicating the two chosen species permanently inhabit at least two of the three sampled streams. On Stuphalletelva no comparable data are available.
Morphometric measurements, larval instars and secondary production
To classify larval instars for analyses of life history patterns and secondary production, each larva of the two considered species was morphometrically analysed according to Schütz and Füreder (2018). Thereby, larval length and the head capsule width were measured for the classification of the four different larval instars. Larval instars were defined by displaying the head capsule width as size frequency histogram where each peak represents an instar, as in Daly (1985) and Schütz and Füreder (2018). As the data were partially not normally distributed, we used Mann–Whitney-U tests (IBM SPSS 23, test considered significant with p < 0.05, if n < 3 no test was run) to compare head capsule widths separately for both species and each larval instar between the three sampled streams. For these analysis samples of the two field trips of each stream were pooled.
We calculated the individual body mass (larval weight) of each larva according to Nolte (1990). We decided to calculate larval weight according to Nolte (1990) instead of drying and weighting the larvae, as larvae could not be separated completely from organic matter without tearing and disrupting them and hence potentially losing parts of the larval body. Contamination with organic matter would also have falsified the results. We compared larval weight separately for both species and each larval instar between the three sampled streams by using Mann–Whitney-U tests (IBM SPSS 23, test considered significant with p < 0.05, if n < 3 no test was run) as data were partially not normally distributed. Data from the two sampling trips were pooled for each stream and each species. Besides the statistics, we also computed means of larval weight over the two samplings for the two species and each larval instar for each stream.
To estimate the productivity of the three sampled streams, linear regression analyses relating larval weight and head capsule width for each of the two considered species separately were plotted for the three streams, allowing estimation of the equations y = a + b*W (µm), with “W” as head capsule width (µm). Data from the two sampling trips were pooled for each stream and each species. Growth of larvae in length proved to be linear as in Schütz and Füreder (2018) and as larval length and weight are linear (Nolte 1990), linear regressions for estimation of larval weight from larval length as measure of productivity were chosen. To give comparable productivity measures of the three streams we calculated the larval secondary production as larval dry weight (m−2) for both species by summing up the larval weights of all individuals from the three replicates from both sampling seasons.
Results
Abiotic stream characterisation
Water temperatures in the kryal river, Bayelva, were quite stable between the two sampling occasions, whereas the mean daily temperatures at the two spring-fed streams, Londonelva and Stuphalletelva, decreased considerably during August (Fig. 2). The seasonal water temperature, defined as mean water temperature between the two benthos sampling dates (22 July–24 August 2016), of Bayelva was 3.01 °C and considerably lower compared to the two rhithral streams Stuphalletelva and Londonelva (Table 1, Fig. 2). Seasonal water temperatures in the two rhithral streams were about twice as warm as in glacier fed Bayelva (Table 1) and hardly differed.
Mean daily water temperatures (°C) with error bars representing the daily range of fluctuation of the three sampled streams between the two sampling occasions (22 July–24 August 2016). Whilst temperatures of the two spring fed streams decreased during summer and hardly differed, glacier fed Bayelva is characterised by comparably low but stable water temperatures throughout the period of record
There was a significantly higher amount of organic matter on the river bottom in Stuphalletelva compared to Londonelva (U = 36.0, Z = 2.882, p = 0.002) and Bayelva (U = 36.0, Z = 2.882, p = 0.002). The amount of organic matter was also significantly higher in Londonelva compared to Bayelva (U = 33.0, Z = 2.402, p = 0.015). Food quality in Londonelva was significantly lower compared to Stuphalletelva (U = 36.0, Z = 2.882; p = 0.002) and Bayelva (U = 21.0, Z = − 2.882, p = 0.002). Food quality at Bayelva was higher than Stuphalletelva, although not significant.
Chironomid head capsule size and larval development
Measurements of head capsule widths allow for clear differentiation of all four larval instars (L1-L4) of D. bohemani and D. aberrata (Table 2). Whereas D. bohemani head capsule widths of the first three larval instars hardly differed among sites, there were significant differences in the fourth larval instar. D. bohemani L4 larvae from Stuphalletelva had significantly wider head capsules compared to Londonelva (U = 7165.0, Z = 3.404, p = 0.003) and Bayelva (U = 139,584.5, Z = 11.296, p < 0.000). L4 larvae from the kryal Bayelva had wider head capsules compared to those from Londonelva, although not significantly. Head capsule widths of D. aberrata differed more clearly between the streams. L1 larvae at Stuphalletelva had significantly wider head capsules compared to those from Londonelva (U = 60.0; Z = 2.766, p = 0.001), the same was true for L2 larvae (U = 463.0, Z = 3.004, p = 0.003) and L3 larvae (U = 2847.4, Z = 5.172, p < 0.001). L4 head capsules of D. aberrata from Londonelva were significantly narrower compared to Bayelva (U = 150.0, Z = -3.287, p = 0.006) and Stuphalletelva (U = 29,033.0, Z = 15.175, p < 0.000). Head capsules from Stuphalletelva were significantly wider compared to those from Bayelva (U = 715.0, Z = 3.682, p < 0.000).
In the kryal Bayelva, the proportion of L4 larvae increased considerably from July to August for both species, indicating a clear maturation of the larval community (Fig. 3). In August, the samples contained no L1 larvae of either species. Whereas no pupae or adults were present in July, August samples contained pupae of both species. Furthermore, flying adults of D. bohemani were caught in August. In the groundwater/snowmelt-fed Londonelva the same trend of a maturing community, indicated by a higher proportion of L4 larvae in August compared to July, was also observed (Fig. 3). However, the proportion of L1 larvae in the August samples increased compared to the July samples. Pupae of both considered species were present in July and August, whereas adults of both species were only caught in August. The percentage of D. bohemani L4 larvae in Stuphalletelva decreased slightly from July to August, whereas clearly more D. aberrata L4 larvae were present in August compared to July (Fig. 3). Proportions of L1 larvae decreased from July until August. Pupae of both species were present in July and August, but flying adults were only caught in August.
Larval weight and secondary production
In general, the weight of the first two larval instars of D. bohemani hardly differed between the three streams (Fig. 4). As the first two larval stages are usually very short, significant size and weight differences cannot be expected and were not apparent. However, the larval weight of L3 larvae from Stuphalletelva (mean: 0.139 mg) was higher as in Bayelva (mean: 0.097 mg; U = 13,751.000, Z = 4.341, p < 0.001) and twice as high compared to Londonelva (mean: 0.067 mg; U = 681.000, Z = 1.949, p < 0.000). The differences between Bayelva and Londonelva were not statistically significant. L4 larvae also had a significantly higher weight in Stuphalletelva (mean: 0.598 mg) compared to Bayelva (mean: 0.299 mg; U = 151,588.000, Z = 13.965, p < 0.000) and Londonelva (mean: 0.282 mg; U = 9467.000, Z = 3.836, p = 0.001). It can easily be seen in Fig. 4 that the majority of L3 and especially L4 larvae found in Stuphalletelva had higher weights compared to the other two streams. Higher mean larval weights in Stuphalletelva are therefore not a result of single very heavy larvae raising the mean but arise from generally bigger and hence heavier larvae. Larvae from Bayelva were slightly heavier compared to Londonelva. Single larvae of Bayleva reached comparable sizes and weights to Stuphalletelva (Fig. 4). This might be due to the high nutrient quality found in Bayelva allowing for distinct larval growth despite the cold water temperatures.
Comparison of larval weights of all four instars of Diamesa bohemani (top graphic) and Diamesa aberrata (bottom graphic) in the three streams, combined for both samplings. White filled symbols as means (plus range) of head capsule width (x-axis) and larval weight (y-axis). If n < 3, white filled symbols represent the weight of single larvae (in this case without error bars). Displayed data points in grey shadings are single measured values
Larval weight of the first two instars of D. aberrata was quite similar at all three streams (Fig. 4). As mentioned above, distinct differences in larval weights in the first two instars cannot be expected. However. L3 larvae from Stuphalletelva (mean: 0.153 mg) were nearly twice as heavy compared to Londonelva (mean: 0.083 mg; U = 2764.000, Z = 4.802, p < 0.000). The single L3 larvae from Bayelva (0.096) was heavier compared to Stuphalletelva, but lighter compared to Londonelva. L4-instars from Londonelva (mean: 0.166 mg) were significantly lighter compared to Bayelva (mean: 0.302 mg; U = 236.000, Z = -2.775, p = 0.006) and Stuphalletelva (mean: 0.731 mg; U = 28,636.000, Z = 14.753, p < 0.000). L4 larvae from Bayelva were, however, also significantly lighter compared to Stuphalletelva (U = 651.000, Z = 2.982, p = 0.003). Differences in larval weight of each individual measured become even more apparent in D. aberrata than in D. bohemani (Fig. 4). L3 and L4 larvae of D. aberrata in Stuphalletelva grew way bigger and heavier compared to the other two streams. While larvae from both species reach comparable size and weight in Stuphalletelva, larvae of D. aberrata in Bayelva and Londonelva remain lighter and especially smaller compared to D. bohemani larvae.
Linear regressions (Table 3) show similar growth rate patterns for both species in the study streams. For both species, growth rates (slope in Table 3) were highest in Stuphalletelva followed by Bayelva and Londonelva. However, the three streams clearly differ in their benthic productivity. Following the growth rates, Stuphalletelva had the highest larval secondary production of all sampled streams (Fig. 5) with 190 mg m−2 larval biomass of D. aberrata and 1209 mg m−2 larval biomass of D. bohemani. Considering D. aberrata, Londonelva produced higher larval biomass (106 mg m−2) compared to Bayelva (4 mg m−2). Secondary production of D. bohemani was higher in Bayelva (136 mg m−2) compared to Londonelva (6 mg m−2).
Discussion
In our study, we found variations in life history patterns, growth rates and larval weight of two chironomid species in three High Arctic streams that considerably varied in terms of water source, water temperature and food quantity and quality. Our results add new information on larval development, suggesting the possibility of univoltinism in High Arctic streams. Furthermore, we were able to show that chironomid larvae in glacier fed streams can have higher growth rates than larvae from the same species in spring/snow fed streams allowing for unexpected productivity of kryal ecosystems.
As expected, water temperatures were lowest in glacier fed Bayelva. Water temperature is known to be an important factor influencing growth and development, especially in cold stream environments (e.g. Caissie 2006; Webb et al. 2008; Blaen et al. 2013). In contrast to alpine regions, where stream interactions with the atmosphere and groundwater have been extensively investigated (e.g. Brown et al. 2006; Ühlinger et al. 2010), temperature dynamics of high latitude streams are still barely understood (Blaen et al. 2013; Docherty et al. 2018a). In the present study stream temperatures follow the trend shown in Blaen et al. (2013) of relatively stable low temperatures in the glacier-fed Bayelva and higher mid-summer temperatures, decreasing during August in the spring/snowmelt-fed streams.
Generally, glacier-fed streams are considered relatively nutrient poor compared to spring-fed streams because of the low water temperatures that inhibit algal growth (e.g. Blaen et al. 2013) and the high instability and abrasion of stone surfaces (Milner and Petts 1994; Brown et al. 2003). Samples from Bayelva followed this general assumption, characterized by the lowest amount of benthic organic matter of all three study streams. Nevertheless, the amount of benthic organic matter in Stuphalletelva was nearly ten times higher than in Londonelva, though both streams are spring/snow fed. This is likely due to the nesting bird colonies on the Stuphallet cliffs (see also Blaen et al. 2013). Thousands of breeding seabirds fertilize the catchment area of Stuphalletelva leading to abundant moss growth below the cliffs and to an abundance of chlorophyll a rich, benthic organic matter in the stream (Blaen et al. 2014). Therefore, chlorophyll a concentrations in Londonelva were considerably lower compared to Stuphalletelva. Interestingly, it was also lower than the glacier-fed Bayelva. Our results are in agreement with chlorophyll a concentrations from a previous study in Bayelva and Stuphalletelva (Blaen et al. 2014) as well as in Londonelva (Lods-Crozet et al. 2007). Thus, benthic organic matter in Londonelva had the significantly lowest food quality of the three sampled streams. Unexpectedly, food quality in Bayelva was highest of the streams, although not significantly different from Stuphalletelva.
Larval community age structure
Water temperature is known to considerably affect the development of benthic invertebrates, including chironomids (e.g. Nolte and Hoffmann 1992; Reynolds and Benke 2005; Hannesdottir et al. 2012; Schütz and Füreder 2018, 2019). Cold adapted species are hypothesized to be especially temperature sensitive (Lillehammer et al. 1989; Knispel et al. 2006; Schütz and Füreder 2018), which is why distinct differences in the larval development strategies in thermally differing, high latitude streams can be expected, although this has never been demonstrated so far. Proportions of larval life stages of D. bohemani and D. aberrata confirm this assumption. The absence of pupae of both species in July at the significantly colder Bayelva, compared to the two groundwater/snowmelt-fed streams where pupae of both species were present, indicate differing development scenarios of both species between the streams. Although D. bohemani caught up in August, indicated by the presence of pupae and adults, as in Londonelva and Stuphalletelva, D. aberrata seemed to be more temperature sensitive, lagging behind the groundwater/snowmelt-fed streams. Our results suggest a stream type dependant flight activity pattern of early flying chironomids at the two rhithral streams beginning in July and a later emergence season in the glacier-fed Bayelva in August, at least for D. bohemani. This leads to omnipresent adult midges during summer, at least at the considered groundwater/snowmelt-fed streams, which is a notable point considering their importance in High Arctic food webs, such as food for nesting birds and their chicks (Hodkinson et al. 1996; Stur and Ekrem 2020). Water temperature obviously shifts larval development of High Arctic chironomids leading to diverging life strategies of the two considered species on small spatial scales. Within only a few kilometres, larval life histories vary considerably leading to a more or less separation of the groundwater/snowmelt-fed stream and glacier-fed stream reproductive cohorts and thus a reduction in gene flow between these stream populations.
The larval stage composition of D. aberrata in the kryal stream is conclusive regarding the life cycle duration as the pattern found suggests an unexpectedly fast larval development. Whereas July samples consisted of L2 and L3 larvae, samples in August contained exclusively L4 larvae and pupae, suggesting a rapid larval development pattern of only some weeks, enabling them to finish the aquatic life stages in late August. Considering the low temperatures in the glacier-fed river, comparable or even faster larval development can be expected for the groundwater/snowmelt-fed streams. Following this rapid larval development, univoltinism in High Arctic chironomids is certainly feasible. Regarding the larval stage composition in the rhithral Londonelva, increasing proportions of L1 larvae from July compared to August suggest a possible second generation of developing larvae during a single season, allowing for the possibility of bivoltinism. Such fast life cycles are surprising but agree with Schütz and Füreder (2018) who also concluded that there were least univoltine life histories in alpine chironomids in a highly glaciated headwater. Furthermore, considering the highly rapid and successful egg development of cold-stenotherm chironomids reported by Schütz and Füreder (2019), univoltinism or even bivoltinism in these High Arctic non-biting midges is unexpected but certainly plausible. These findings add important information on the life histories and development patterns of Arctic invertebrates as their life cycles were shown to be very slow due to the low temperatures in high latitudinal environments, leading to perennial development scenarios (e.g. Danks 1992). For example, the life cycle of two arctic Chironomus species (Chironomidae, Diptera) inhabiting tundra ponds was shown to take seven years (Butler et al. 1981; Butler 1982). Nevertheless, our results are confirmed by Danks (2007) who reported that several High Arctic insect taxa completed their life cycles within one year. Some species, especially those inhabiting the High Arctic streams are obviously characterized by comparably fast life cycles like High Alpine species (e.g. Schütz and Füreder 2018, 2019). This could be an adaption towards the very short snow and ice-free growing season during summer and could be related to differing freezing tolerances of the various life cycle stages. Our study species are obviously able to complete their life cycle within a short period of non-freezing temperatures, free flowing streams and continuous daylight.
Our results lead to conclusions on the life cycle patterns and development histories of the studied species during the short Arctic summer. However, hardly anything is known about the overwintering of Arctic insects, especially cold adapted chironomids. Danks (2007) is one of the few studies reporting on the life of aquatic insects during winter. Though little is known on the winter conditions in High Arctic stream ecosystems, it can be assumed that chironomid larvae have to face very low or freezing temperatures for a long time during winter and are therefore dormant (Danks 2007). Following available studies from cold freshwater ecosystems, two different overwintering strategies seem possible. As larvae of some cold adapted chironomid species are known to be freezing tolerant (e.g. Danks 1971; Olsson 1981; Lencioni 2004; Bouchard et al. 2006), overwintering as larvae seems plausible for the studied High Arctic chironomids. However, as Olsson (1981) reported quite low survival rates of overwintering chironomid larvae, a maybe more promising strategy to survive freezing temperatures is to overwinter in the egg stage (Danks 2007). This is known from mosquitos (e.g. Copeland and Craig 1990) and mayflies (e.g. Giberson and Galloway 1985) including an Arctic Baetis species (Giberson et al. 2007) and is also conceivable for High Arctic chironomids. To finally conclude on the overwintering and hence the life histories of High Arctic chironomids and document the possibility of univoltine or bivoltine life cycles, further studies concentrating on the overwintering of High Arctic chironomids are necessary as up to now only assumptions can be made on this substantial part of the chironomid life cycle.
Stream productivity
Information on larval size and growth in cold streams has only recently become available, confirming highly adapted, cold-stenotherm species a hitherto unexpected growth rate resulting in larval sizes comparable to low-land streams (Niedrist and Füreder 2018; Schütz and Füreder 2018). Thus, nutrient availability and not temperature proved to be the limiting growth factor for benthic larvae (Schütz and Füreder 2018). The cited studies are, however, concentrated on high altitude streams in the European alps, only allowing for limited conclusions on the larval growth and productivity in High Arctic streams. The extremely short growing season of only a few weeks for benthic invertebrates in high latitude streams raises the question if life strategies of chironomid larvae in the High Arctic streams allow for distinct larval growth rates or if life histories are programmed for the quickest possible development.
The noticeably different larval size (head capsule width) and weight patterns in the streams considered in the present study indicate that fast life cycles by concurrently distinct larval growth rates are not mutually exclusive life cycle strategies in High Arctic streams. Larval head capsule size and weight of both Diamesa species were lowest in groundwater/snowmelt-fed Londonelva, followed by kryal Bayelva and Stuphalletelva. Despite the higher nutrient availability and the more benign general abiotic conditions (higher water temperature, less turbidity) in Londonelva compared to the glacially influenced Bayelva larvae of both species remained smaller and lighter in the spring-fed stream. This unexpected pattern can only be explained by the low food quality of the benthic organic matter in Londonelva. Though food availability in Bayelva is lowest of all three considered streams the food quality of the glacially influenced stream is best of the three study streams leading to significantly larger and heavier larvae and an increased productivity in the glacier-fed stream. This goes along with the findings of Schütz and Füreder (2018) reporting on distinct larval growth in a highly glaciated headwater. The best compromise for most efficient larval growth was found in rhithral Stuphalletelva. Though food quality was somewhat lower compared to Bayelva, larvae in the nutrient enriched groundwater/snowmelt-fed stream grew to the largest size, resulting in the highest growth rates and thus the highest productivity. The combination of a surplus of nutrients of reasonable quality, comparably high water temperatures and low turbidity are obviously the key factors for fast larval development through outstanding larval growth. The nesting bird colony in the Stuphalletelva catchments (Blaen et al. 2013) obviously does not only fertilize the catchment area, but also leads to an enhanced instream primary and secondary production.
The high chironomid larval growth rates in cold-streams was assumed to be contradictory. However, recent studies have demonstrated that highly adapted chironomid species can reach unexpected larval sizes and weight in high altitudinal alpine streams, enriching the food chain of these harsh freshwaters at the initial link (e.g. Niedrist and Füreder 2017, 2018; Schütz and Füreder 2018). The present study is the first attempt to quantify the larval growth and hence the secondary production of High Arctic streams, demonstrating remarkable larval sizes subjected to instream abiotic conditions. Nutrient availability and in particular food quality obviously trigger increased benthic larval size independently of the stream type. Considering future climate change effects with increasing temperatures potentially leading to higher instream primary production might therefore lead to distinct larval growth patterns and enhanced storage of allochthonous material in high latitude streams.
The present study is an important step towards the understanding of larval development and growth in High Arctic streams. Nevertheless, many questions remain regarding the dynamics of chironomid life cycles. A prolonged sampling season, following chironomid development throughout the whole snow and ice-free season would allow for improved conclusions on the species’ life histories and adaptational abilities to the predominant harsh abiotic conditions. Furthermore, nothing is known about chironomids during winter. The two species in the present study belong to the family of Diamesa which is known to be especially cold-stenotherm and adapted to harsh conditions. In addition to these particularly robust species, representatives of the subfamily Orthocladiinae also inhabit High Arctic streams, often at high densities (own observations; see also Lods-Crozet et al. 2007; Marziali et al. 2009). Species from this subfamily may have different life cycle strategies leading to alternative development patterns.
Nevertheless, the detected pattern of distinct chironomid larval growth at environmental conditions considered as at or near the physiological limit of benthic life (Danks et al. 1994) were unexpected and add new knowledge on the adaptabilities of cold-stenotherm chironomids to their harsh living conditions. Glacially influenced streams may well also be more productive than assumed due to unexpected high growth rates of chironomid larvae, obviously allowing for larval growth respectively secondary production comparable to nutrient poor groundwater/snowmelt-fed streams.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Blaen PJ, Hannah DM, Brown LE, Milner AM (2013) Water temperature dynamics in High Arctic river basins. Hydrol Process 27:2958–2972
Blaen PJ, Brown LE, Hannah DM, Milner AM (2014) Environmental drivers of macroinvertebrate communities in High Arctic rivers (Svalbard). Freshw Biol 59:378–391
Bouchard RW, Carillo MA, Kells SA, Ferrington LC (2006) Freeze tolerance in larvae of winter-active Diamesa mendotae Muttkowski (Diptera: Chironomidae), a winter-emerging aquatic insect. Hydrobiol 568:403–416
Brittain JE, Milner AM (2001) Ecology of glacier-fed rivers: current status and concepts. Freshw Biol 46:1571–1578
Brown LE, Hannah DM, Milner AM (2003) Alpine stream habitat classification: an alternative approach incorporating the role of dynamic water source contributions. Arct Antarct Alp Res 35:313–322
Brwon LE, Hannah DM, Milner AM (2006) Thermal variability and stream flow permanency in an alpine river system. River Res Appl 22:491–501
Butler MG (1982) A 7-year life cycle for two Chironomus species in arctic Alaskan tundra ponds (Diptera: Chironomidae). Can J Zool 60:58–70
Butler MG, Miller MC, Mozley S (1981) Macrobenthos. In: Hobbie JE (ed) Limnology of tundra ponds (Insecta, Diptera, Chironomidae). Dowden, Hutchinson and Ross, Stroudsburg, pp 297–339
Caissie D (2006) The thermal regime of rivers: a review. Freshw Biol 51:1389–1406
Copeland RS, Craig GB (1990) Cold hardiness of tree-hole mosquitos in the Great Lakes region of the United States. Can J Zool 68:1307–1314
Coulson SJ, Convey P, Aakra K, Aarvik L, Ávila-Jimenéz ML, Babenko A, Biersma E, Boström S, Brittain JE (2014) The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea, Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biol Biochem 68:440–470
Daly HV (1985) Insect morphometrics. Annu Rev Entomol 30:415–438
Danks H (1971) Overwintering of some north temperate and arctic Chironomidae: II. Chironomid biology. Can Entomol 103:1875–1910
Danks H (1992) Long life cycles in insects. Can Entomol 123:23–40
Danks H (2007) How aquatic insects live in cold climates. Can Entomol 139:443–471
Danks HV, Kukal O, Ring RA (1994) Insect cold-hardiness: insights from the arctic. Arctic 47:391–404
Docherty CL, Hannah DM, Riis T, Lund M, Abermann J, Milner AM (2018a) Spatio-temporal dynamics of macroinvertebrate communities in northeast Greenlandic snowmelt streams. Ecohydrol. https://doi.org/10.1002/eco.1982
Docherty CL, Hannah DM, Riis T, Leth SR, Milner AM (2018b) Longitudinal distribution of macroinvertebrates in snowmelt streams in northeast Greenland: understanding biophysical controls. Polar Biol 41:1567–1580
Ferrarese U, Rossaro B (1981) Chironomidi 1 (Diptera Chironomidae: Generalita, Diamesinae, Prodiamesinae). Guide per il riconoscimento delle specie animali delle acque interne italiane, 12. Consiglio Nazionale delle Ricerche, Verona
Friberg N, Milner AM, Svendsen LM, Lindegaard C, Larsen SE (2001) Macroinvertebrate stream communities along regional and physico-chemical gradients in Western Greenland. Freshw Biol 46:1753–1764
Fuller RL, Kennedy BP, Nielsen C (2004) Macroinvertebrate response to algal and bacterial manipulations in streams. Hydrobiologia 523:113–126
Füreder L (2007) Life at the edge: habitat condition and bottom fauna of Alpine running waters. Int Rev Hydrobiol 92:491–513
Füreder L, Niedrist GH (2020) Glacial stream ecology: structural and functional assets. Water 12:376. https://doi.org/10.3390/w12020376
Giberson DJ, Galloway TD (1985) Life history and production of Ephron album (Say) (Ephemeoptera: Polymitarcidae) in the Valley River, Manitoba. Can J Zool 63:1668–1674
Giberson DJ, Burian SK, Shouldice M (2007) Life history of northern mayfly, Baetis bundyae (Ephemeroptera) in Rankin Inlet, Nunavut Territory, Canada, with updates to the mayflies of Canada list. Can Entomol 139:628–642
Hannesdottir ER, Gislason GM, Olafsson JS (2012) Life cycles of Eukiefferiella claripennis (Lundbeck 1898) and Eukiefferielle minor (Edwards 1929) (Diptera: Chironomidae) in spring-fed streams of different temperatures with reference to climate change. Fauna Norv 31:35–46
Hodkinson ID, Coulson SJ, Webb NR, Block W, Strathdee AT, Bale JS, Worland MR (1996) Temperature and the biomass of flying midges (Diptera: Chironomidae) in the High Arctic. Oikos 75:241–248
Janecek FR (1998) Fauna Aquatica Austriaca, Taxonomie und Ökologie aquatischer wirbelloser Organismen, Diptera: Chironomidae (Zuckmücken). Universität für Bodenkultur, Abteilung Hydrobiologie, Wien
Knispel S, Sartori M, Brittain JE (2006) Egg development in the mayflies of a Swiss glacial floodplain. J N Am Benthol Soc 25(2):430–443
Langton PH (1991) A key to pupal exuviae of West Palaearctic Chironomidae. Huntingdon, Cambridgeshire
Lencioni V (2004) Survival strategies of freshwater insects in cold environments. J Limnol 63:45–55
Langton PH, Pinder LCV (2007) Keys to the adult male Chironomidae of Britain and Ireland (2 Volumes). Freshwater Biological Association, Ambleside
Lillehammer A, Brittain JE, Saltveit SJ, Nielsen PS (1989) Egg development, nymphal growth and life cycle strategies in Plecoptera. Ecography 12(2):173–186
Lods-Crozet B, Lencioni V, Olafsson J, Snook D, Velle G, Brittain JE, Castella E, Rossaro B (2001) Chironomid (Diptera: Chironomidae) communities in six European glacier-fed streams. Freshw Biol 46:1791–1809
Lods-Crozet B, Lencioni V, Brittain JE, Marziali L, Rossaro B (2007) Contrasting chironomid assemblages in two High Arctic streams on Svalbard. Fundam Appl Limnol 170:211–222
Lorenzen CJ (1967) Determination of chlorophyll and pheo-pigment: spectrophotometric equations. Limnol Oceanogr 12:343–346
Makarchenko EA, Makarchenko MA (1999) Chironomidae. In: Tsalolokhin SJ (ed) Key to freshwater invertebrates of Russia and adjacent lands, vol 210–295. Zoological Institute RAS, St. Petersburg, pp 670–857
Marziali L, Gozzini M, Rossaro B, Lencioni V (2009) Drift patterns of Chironomidae (Insecta, Diptera) in an arctic stream (Svalbard Islands): and experimental approach. Studi Trent Sci Nat 84:87–96
Milner AM, Petts GE (1994) Glacial rivers: physical habitat and ecology. Freshw Biol 32:295–307
Niedrist GH, Füreder L (2017) Trophic ecology of alpine stream invertebrates: current status and future research needs. Freshw Sci 36:466–478
Niedrist GH, Füreder L (2018) When the going gets tough, the tough get going: the enigma of survival strategies in harsh glacial stream environments. Freshw Biol 63(10):1260–1272. https://doi.org/10.1111/fwb.13131
Nolte U (1990) Chironomid biomass determination from larval shape. Freshw Biol 24:443–451
Nolte U, Hoffmann T (1992) Fast life in cold water: Diamesa incallida (Chironomidae). Ecography 15:25–30
Olsson TI (1981) Overwintering of benthic macroinvertebrates in ice and frozen sediment in a north Swedish river. J Exp Biol 201:1585–1594
Prowse TD, Wrona FJ, Reist JD, Gibson JJ, Hobbie JE, Levesque LMJ, Vincent WF (2006) Climate change effests on hydroecology of arctic freshwater ecosystems. AMBIO: J Hum Environ 35:347–358
Reynolds SK, Benke AC (2005) Temperature-dependent growth rates of larval midges (Diptera: Chionomidae) from a southeastern US stream. Hydrobiologia 544:69–75
Robinson CT, Thompson C, Freestone M (2014) Ecosystem development of streams lengthened by rapid glacial recession. Fundam Appl Limnol 185:235–246
Rossaro B, Lencioni V (2015a) A key to larvae of species belonging to the genus Diamesa from Alps and Apennines (Italy). Eur J Environ Sci 5:62–79
Rossaro B, Lencioni V (2015b) A key to larvae of Diamesa Meigen, 1835 (Diptera: Chironomidae), well known as adult males and pupae from Alps (Europe). J Entomol Acarol Res 47:123–138
Sand K, Brittain JE (2009) Life cycle shifts in Baetis rhodani (Ephemeroptera) in the Norwegian mountains. Aquat Insects 31:83–291
Schmid PE (1993) Wasser und Abwasser. A key to the larval Chironomidae and their instars from Austrian Danube region streams and rivers with particular reference to a numerical taxonomic approach. Fed Inst Water Qual Wien Supplementary 3/93:1–514
Schütz SA, Füreder L (2018) Unexpected patterns of larval size in an extreme environment: a highly glaciated, alpine stream. Hydrobiologia 820:49–63
Schütz SA, Füreder L (2019) Egg development and hatching success in alpine chironomids. Freshw Biol 64(4):685–696
Serra-Tosio B (1971) Contribution l’étude taxonomique, phylogénétique, biogéo-graphique et écologique des Diamesini (Diptera, Chironomidae) d'Europe. Dissertation, University of Grenoble
Steffan AW (1971) Chironomid (Diptera) biocenoses in Scandinavian glacier brooks. Can Entomol 103:477–486
Stur E, Ekrem T (2020) The Chironomidae (Diptera) of Svalbard and Jan Mayen. Insects. https://doi.org/10.3390/insects11030183
Svenning MA, Klemetsen A, Olsen T (2007) Habitat and food choice of Arctic charr in Linnevatn on Spitsbergen, Svalbard: the first year-round investigation in a High Arctic lake. Ecol Freshw Fish 16:70–77
Ühlinger U, Robinson CT, Hieber M, Zah R (2010) The physico-chemical habitat template for periphyton in alpine glacial streams under a changing climate. Hydrobiologia 657:107–121
Vincent WF, Callaghan TV, Dahl-Jensen D, Johansson M, Kovacs KM, Mechel C, Prowse T, Reist JD, Sharp M (2011) Ecological implications of changes in the arctic cryosphere. AMBIO: J Hum Environ 40:87–99
Webb BW, Hannah DM, Moore RD, Brown LE, Nobilis F (2008) Recent advances in stream and river temperature research. Hydrol Process 918:902–918
Wiederholm T (1983) Chironomidae of the Holarctic region. Key and diagnoses. Part I: Larvae Ent Scand Suppl 19:1–457
Wiederholm T (1989) Chironomidae of the Holarctic region. Keys and diagnoses. Part III: Adult males. Ent Scand Suppl 34:532
Wrona FJ, Prowse TD, Reist JD, Hobbie JE, Levesque LMJ, Vincent WF (2006) Climate change effects on aquatic biota, ecosystem structure and function. AMBIO: J Hum Environ 35:359–369
Acknowledgements
We thank the University of Innsbruck for providing SAS with a Ph.D. student grant. Furthermore, we want to thank the Research Council of Norway and the Svalbard Science Forum for awarding SAS an Arctic Field Grant (AFG) to cover the costs of the two field trips to Ny-Ålesund. JEB was supported by the Natural History Museum, University of Oslo and the Norwegian Water Resources and Energy Directorate and LF by the Austrian Academy of Sciences and the University of Innsbruck (project HASTE – High Arctic Stream Ecosystems). Moreover, we thank Claudia Breitschopf for her help in the field and laboratory.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. SAS received a Ph.D. student grant from the University of Innsbruck. The Research Council of Norway and the Svalbard Science Forum awarded SAS an Arctic Field Grant (AFG) to cover the costs of the two field trips to Ny-Ålesund.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by all authors. SAS did laboratory analysis, species determination, all further analysis, statistics and wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript and contributed equally to the final manuscript. All authors read and approved to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
No ethical approval is required for the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Research involved in human and animal rights
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schütz, S.A., Brittain, J.E. & Füreder, L. Diverging life cycle patterns of two Diamesa species (Diptera, Chironomidae) in High Arctic streams, Svalbard. Polar Biol 45, 285–296 (2022). https://doi.org/10.1007/s00300-021-02987-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02987-1