Abstract
Key message
Expression of TaRUB1 gene in Arabidopsis thaliana elevates the level of disease-related genes in response to pathogen invasion through the accumulation of callose, necrotic cells, and the outbreak of ROS.
Abstract
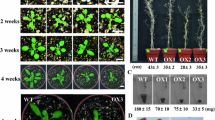
Ubiquitin (Ub) and ubiquitin-like proteins are highly conserved in sequence and can covalently bind and modify many intracellular proteins which can be recognized and degraded by 26S proteasome. Post-translational modification of proteins has become a hot research spot today. In the previous study, a cDNA of related-to-ubiquitin protein belonged to ubiquitin-like proteins, whose spatial structure comprised Ub and NEDD8, was obtained from wheat SN6306 by suppression-subtractive hybridization and was named TaRUB1. TaRUB1 is induced by wheat powdery mildew and significantly upregulated in resistant wheat SN6306. In this study, heterologous expression of TaRUB1 in A. thaliana was used to study the function of this gene in response to pathogen Pseudomonas syringae pv. Tomato DC3000 (Pst DC3000). Transgenic A. thaliana showed relatively fewer disease symptoms, accompanied by common inhibition of living body parasitic defense responses, accumulation of more callose and reactive oxygen species (ROS), and concentrated cell death, simultaneously antioxidant enzyme activities of superoxide dismutase, peroxidase, catalase, and ascorbate peroxidase were higher than those in wild-type (WT) plant after infection with Pst DC3000. Meanwhile, hypersensitive cell death, which was possibly ROS burst, was also observed in transgenic A. thaliana. By quantitative reverse transcription-polymerase chain reaction analysis, some marker genes for hypersensitive response showed significantly higher transcriptional expression level in transgenic A. thaliana, which activates system-acquired resistance, than that of WT plants. Heterologous expression of TaRUB1 can significantly enhance resistance to Pst DC3000 in A. thaliana, suggesting that TaRUB1 is related to plant disease resistance.





Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- dpi:
-
Days post-inoculation
- DUB:
-
Deubiquitinase
- DAB:
-
Diaminobenzidine
- ETI:
-
Effector trigger immunity
- H2O2 :
-
Hydrogen peroxide
- HR:
-
Hypersensitive response
- ISR:
-
Induced systemic resistance
- NBT:
-
Nitroblue tetrazolium
- ROS:
-
Reactive oxygen species
- PAMPs:
-
Pathogen-associated molecular patterns
- PRRs:
-
Pattern-recognition receptors
- PTI:
-
Pattern-triggered immunity
- POD:
-
Peroxidase
- Pst DC3000:
-
Pseudomonas syringae pv. tomato DC3000
- RUB:
-
Related-to-ubiquin
- SA:
-
Salicylic acid
- O2 .− :
-
Superoxide radicals
- SAR:
-
System--acquired resistance
- Ub:
-
Ubiquitin
References
Bigeard J, Colcombet J, Hirt H (2015) Signaling mechanisms in pattern-triggered immunity (PTI). Mol Plant 8:521–539
Dong JX, Chen CH, Chen ZX (2003) Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51:21–37
Dunand C, Crevecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
Feng Y, Zhang M, Guo Q, Wang G, Gong J, Xu Y, Wang W (2014) Manipulation of monoubiquitin improves chilling tolerance in transgenic tobacco (Nicotiana tabacum). Plant Physiol Biochem 75:138–144
Gidekel M, Destefano-Beltrán L, Garcia P, Fuentes L, Alberdi M, Bravo L, Corcuera L, Gutierrez A (2003) Identification and characterization of three novel cold- acclimation responsive genes from the extremophile hair grass Deschampsia antarctica Desv. Extremophiles 7:459–469
Hauck P, Thilmony R, He SY (2003) A Pseudomonas syringae type III effector suppresses cell wall-based extra-cellular defense insusceptible Arabidopsis plants. Proc Natl Acad Sci 100:8577–8582
Huang DT, Walden H, Duda D, Schulman BA (2004) Ubiquitin-like protein activation. Oncogene 23:1985–1971
Katagiri F, Thilmony R, He SY (2002) The Arabidopsis thaliana–Pseudomonas syringae interaction. Arabidopsis Book Am Soc Plant Biol 1:e0039
Kim DS, Hwang BK (2014) An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signaling of the defense response to microbial pathogens. J Exp Bot 65:2295–2306
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Lamb C, Dixon RA (1997) The oxidative bust in plant disease. Plant Mol Biol 48:251–275
Lebel E, Heifetz P, Thorne L, Uknes S, Ryals J, Ward E (1998) Functional analysis of regulatory sequences controlling PR-1 gene expression in Arabidopsis. Plant J 16:223–233
Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dang JL, Dietrich RA (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26:403–410
Nicaise V, Roux M, Zipfel C (2009) Recent advances in PAMP-triggered immunity against bacteria: pattern recognition on receptors watch over and raise the alarm. Plant Physiol 150:1638–1647
Nimchuk Z, Eulgem T, Holt Iii BF, Dangl JL (2003) Recognition and response in the plant immune system. Annu Rev Genet 37:579–609
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
O’Brien J a, Daudi A, Butt VS, Bolwell GP (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779
Osaka F, Saeki M, Katayama S, Aida N, Toh-E A, Kominami K, Toda T, Suzuki T, Chiba T, Tanaka K, Kato S (2000) Covalent modifier NEDD8 is essential for SCF ubiquitin-ligase in fission yeast. EMBO J 19:3475–3484
Peng HC, Kaloshian I (2014) The tomato leucine-Rich repeat receptor-like kinases SlSERK3A and SlSERK3B have overlapping functions in bacterial and nematode innate immunity. PLoS One 9:e93302
Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1:404–411
Sacco JJ, Coulson JM, Clague MJ, Urbe´S (2010) Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life 62:140–157
Schenk ST, Schikora A (2015) Staining of callose depositions in root and leaf tissues. Bio-Protocol 5:e1429
Seo PJ, Park CM (2010) MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol 186:471–483
Seo PJ, Lee AK, Xiang FN, Park CM (2008) Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiol 49:334–344
Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Cammue BPA, Broekaert WF (1998) Separate jasmonate dependent and salicylic acid-dependent defense response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95:15107–15111
van Wees S (2008) Phenotypic analysis of Arabidopsis mutants: trypan blue stain for fungi, oomycetes, and dead plant cells. Cold Spring Harb Protoc 3(8):1–2
Vierstra RD, Callis J (1999) Polypeptides tags, ubiquitious modifiers for plant protein regulation. Plant Mol Biol 41:435–442
Wan JR, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey MG, Stacey G (2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20:471–481
Wang GK, Zhang M, Gong JF, Guo QF, Feng YN, Wang W (2012) Increased gibberellin contents contribute to accelerated growth and development of transgenic tobacco overexpressing a wheat ubiquitin gene. Plant Cell Rep 31:2215–2227
Yang W, Dong R, Liu L, Hu ZB, Jing L, Wang Y, Ding XH, Chu ZH (2016) A novel mutant allele of SSI2 confers a better balance between disease resistance and plant growth inhibition on Arabidopsis thaliana. BMC Plant Biol 16:208
Zhang J, Guo QF, Feng YN, Li F, Gong JF, Fan ZY, Wang W (2012) Manipulation of monoubiquitin improves salt tolerance in transgenic tobacco. Plant Biol 14:315–324
Zhang Y, Feng DS, Bao YG, Ma X, Yin N, Xu JQ, Wang HG (2013) A novel wheat related-to-ubiquitin gene TaRUB1 is responsive to pathogen attack as well as to both osmotic and salt stress. Plant Mol Biol Rep 31:151–159
Zhang M, Kang HH, Zhang GQ, Chen YH, Kong XZ, Guo QF, Wang W (2015) Overexpression of TaUb2 enhances disease resistance to Pseudomonas syringae pv. tomato DC3000 in tobacco. Physiol Mol Plant P 90:98–104
Zhu JK (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4:401–406
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0102000 to HGW) and the National Natural Science Foundation of China (nos. 31171552 and 31771777 to DSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Chun-Hai Dong.
Rights and permissions
About this article
Cite this article
Yang, Y., Wang, W., Xu, T. et al. Heterologous expression of wheat TaRUB1 gene enhances disease resistance in Arabidopsis thaliana . Plant Cell Rep 36, 1985–1994 (2017). https://doi.org/10.1007/s00299-017-2221-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2221-1