Abstract
Key message
Three wild species exhibited a significant reduction in antioxidants throughout the cryopreservation protocol, whilst the half-cell reduction potential became more oxidised. Antioxidant content recuperated in recovering shoot tips.
Abstract
Cryopreservation is the most efficient and cost-effective long-term storage solution for the conservation of a wide range of plant species and material. Changes in the levels of antioxidants during the process of cryopreservation are known to reduce post-cryogenic survival due to oxidative stress. Low-molecular-weight thiols (cysteine, γ-glutamylcysteine, and glutathione) and ascorbic acid, which represent the two major water-soluble antioxidants in plants, were analysed at specific stages during cryopreservation of shoot tip material of three native Australian plant species [Anigozanthos viridis (Haemodoraceae), Lomandra sonderi (Asparagaceae), and Loxocarya cinerea (Restionaceae)] to quantify the oxidative stress experienced during cryopreservation. Post-cryogenic regeneration of shoot tips was greatest in A. viridis (78%) followed by L. sonderi (50%), whilst L. cinerea did not show any post-cryogenic regeneration. The application of a 3-week cold (5 °C) preconditioning regime, commonly used to increase post-cryogenic survival, resulted in significantly lower post-cryogenic regeneration for A. viridis (33%), but had little effect on the other two species. Total antioxidant concentration in shoot material decreased significantly with each step throughout the cryopreservation process, particularly in the cryoprotection and washing stages. Antioxidant levels in shoot tips then increased during the subsequent 7-day post-cryopreservation recovery period, with the greatest increase measured in A. viridis. Concentrations of thiols and their corresponding disulphides were used to calculate the corresponding half-cell reduction potentials, whereby the ability of these plant species to maintain a strong reducing environment in shoot tissues throughout the cryopreservation protocol was found to correlate with post-cryogenic survival.

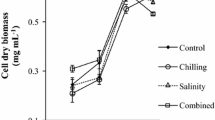
Adapted from Kaczmarczyk et al. (2012); originally published under CC BY 3.0 license. Available from: doi:10.5772/32860





Similar content being viewed by others
Abbreviations
- AsA:
-
Ascorbic acid
- BM:
-
Basal medium
- CPAs:
-
Cryoprotective agents
- Cys:
-
Cysteine
- DW:
-
Dry weight
- FW:
-
Fresh weight
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulphide
- γ-Glu-Cys:
-
Gamma-glutamylcysteine
- LMW:
-
Low molecular weight
- LN:
-
Liquid nitrogen
- ROS:
-
Reactive oxygen species
References
Bachiri Y, Bajon C, Sauvanet A, Gazeau C, Morisset C (2000) Effect of osmotic stress on tolerance of air-drying and cryopreservation of Arabidopsis thaliana suspension cells. Protoplasma 214:227–243
Bajaj YPS (1995) Cryopreservation of plant cell, tissue, and organ culture for the conservation of germplasm and biodiversity. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 32. Springer, New York, pp 1–18
Barraco G, Chatelet P, Balsemin E, Decourcelle T, Sylvestre I, Engelmann F (2012) Cryopreservation of Prunus cerasus through vitrification and replacement of cold hardening with preculture on medium enriched with sucrose and/or glycerol. Sci Hortic 148:104–108
Benson EE, Bremner D (2004) Oxidative stress in the frozen plant: a free radical point of view. In: Fuller BJ, Lane N, Benson EE (eds) Life in the frozen state. CRC Press, Boca Raton, pp 206–241
Berjak P, Pammenter N (2014) Cryostorage of germplasm of tropical recalcitrant-seeded species: approaches and problems. Int J Plant Sci 175:29–39
Berjak P, Varghese B, Pammenter N (2011) Cathodic amelioration of the adverse effects of oxidative stress accompanying procedures necessary for cryopreservation of embryonic axes of recalcitrant-seeded species. Seed Sci Res 21:187–203
Birtic S, Colville L, Pritchard HW, Pearce SR, Kranner I (2011) Mathematically combined half-cell reduction potentials of low-molecular-weight thiols as markers of seed ageing. Free Radic Res 45:1093–1102
Canepa P, Panta A, Tay D (2011) The effect of antioxidants on the cryopreservation recovery of two potato cultivars following post-thawing culture. Acta Hort 908:101–105
Chang Y, Barker RE, Reed BM (2000) Cold acclimation improves recovery of cryopreserved grass (Zoysia and Lolium sp.). CryoLetters 21:107–116
Chen H, Osuna D, Colville L, Lorenzo O, Graeber K, Küster H, Leubner-Metzger G, Kranner I (2013) Transcriptome-wide mapping of pea seed ageing reveals a pivotal role for genes related to oxidative stress and programmed cell death. PLoS One 8:e78471
Chen G, Ren L, Zhang J, Reed BM, Zhang D, Shen X (2015) Cryopreservation affects ROS-induced oxidative stress and antioxidant response in Arabidopsis seedlings. Cryobiology 70:38–47
Colville L, Kranner I (2010) Desiccation tolerant plants as model systems to study redox regulation of protein thiols. Plant Growth Regul 62:241–255
Department of Parks and Wildlife (2014) Wildlife Conservation (Rare Flora) Notice. https://www.dpaw.wa.gov.au/plants-and-animals/threatened-species-and-communities/threatened-plants
Dussert S, Chabrillange N, Montillet JL, Agnel JP, Engelmann F, Noirot M (2003) Basis of coffee seed sensitivity to liquid nitrogen exposure: oxidative stress or imbibitional damage? Physiol Plant 119:534–543
Fadzillah NM, Gill V, Finch RP, Burdon RH (1996) Chilling, oxidative stress and antioxidant responses in shoot cultures of rice. Planta 199:552–556
Fang JY, Wetten A, Johnston J (2008) Headspace volatile markers for sensitivity of cocoa (Theobroma cacao L.) somatic embryos to cryopreservation. Plant Cell Rep 27:453–461
Folgado R, Panis B, Sergeant K, Renaut J, Swennen R, Hausman J-F (2015) Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 71:432–441
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Fujikawa S, Jitsuyama Y, Kuroda K (1999) Determination of the role of cold acclimation-induced diverse changes in plant cells from the viewpoint of avoidance of freezing injury. J Plant Res 112:237–244
Funnekotter B, Kaczmarczyk A, Turner SR, Bunn E, Zhou W, Smith S, Flematti G, Mancera RL (2013) Acclimation-induced changes in cell membrane composition and influence on cryotolerance of in vitro shoots of native plant species. Plant Cell Tissue Organ Cult 114:83–96
Funnekotter B, Sortey A, Bunn E, Turner S, Mancera R (2016) Influence of abiotic stress preconditioning on antioxidant enzymes in shoot tips of Lomandra sonderi (Asparagaceae) prior to cryostorage. Aust J Bot 64:260–268
Gupta S, Reed BM (2006) Cryopreservation of shoot tips of blackberry and raspberry by encapsulation–dehydration and vitrification. CryoLetters 27:29–42
Harding K, Johnston JW, Benson EE (2009) Exploring the physiological basis of cryopreservation success and failure in clonally propagated in vitro crop plant germplasm. Agric Food Sci 18:103–116
Hincha D, Zuther E (2014) Introduction: plant cold acclimation and freezing tolerance. In: Hincha DK, Zuther E (eds) Plant cold acclimation. Springer, New York, pp 1–6
Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annu Rev Ecol Evol Syst 35:623–650
Hughes ZE, Mancera RL (2013) Molecular dynamics simulations of mixed DOPC–β-sitosterol bilayers and their interactions with DMSO. Soft Matter 9:2920–2935
Hughes ZE, Mancera RL (2014) Molecular mechanism of the synergistic effects of vitrification solutions on the stability of phospholipid bilayers. Biophys J 106:2617–2624
Hughes ZE, Mark AE, Mancera RL (2012) Molecular dynamics simulations of the interactions of DMSO with DPPC and DOPC phospholipid membranes. J Phys Chem B 116:11911–11923
Johnston JW, Harding K, Benson EE (2007) Antioxidant status and genotypic tolerance of Ribes in vitro cultures to cryopreservation. Plant Sci 172:524–534
Kaczmarczyk A, Shvachko N, Lupysheva Y, Hajirezaei MR, Keller ER (2008) Influence of alternating temperature preculture on cryopreservation results for potato shoot tips. Plant Cell Rep 27:1551–1558
Kaczmarczyk A, Turner SR, Bunn E, Mancera RL, Dixon KW (2011) Cryopreservation of threatened native Australian species—what have we learned and where to from here? In Vitro Cell Dev Biol Plant 47:17–25
Kaczmarczyk A, Funnekotter B, Menon A, Phang PY, Al-Hanbali A, Bunn E, Mancera RL (2012) Current issues in plant cryopreservation. In: Katkov II (ed) Current frontiers in cryobiology. InTech, Croatia, pp 417–438
Kaczmarczyk A, Funnekotter B, Turner SR, Bunn E, Bryant G, Hunt TE, Mancera RL (2013) Development of cryopreservation for Loxocarya cinerea—an endemic Australian plant species important for post-mining restoration. CryoLetters 34:508–519
Kamata T, Uemura M (2004) Solute accumulation in wheat seedlings during cold acclimation: contribution to increased freezing tolerance. CryoLetters 25:311–322
Kocsy G, Szalai G, Vágújfalvi A, Stéhli L, Orosz G, Galiba G (2000) Genetic study of glutathione accumulation during cold hardening in wheat. Planta 210:295–301
Kocsy G, Galiba G, Brunold C (2001) Role of glutathione in adaptation and signalling during chilling and cold acclimation in plants. Physiol Plant 113:158–164
Kranner I (1998) Determination of glutathione, glutathione disulphide and two related enzymes, glutathione reductase and glucose-6-phosphate dehydrogenase, in fungal and plant cells. In: Varma A (ed) Mycorrhiza manual. Springer, Berlin, pp 227–241
Kranner I, Birtić S, Anderson KM, Pritchard HW (2006) Glutathione half-cell reduction potential: a universal stress marker and modulator of programmed cell death? Free Radic Biol Med 40:2155–2165
Leunufna S, Keller ER (2005) Cryopreservation of yams using vitrification modified by including droplet method: effects of cold acclimation and sucrose. CryoLetters 26:93–102
Lynch PT, Siddika A, Johnston JW, Trigwell SM, Mehra A, Benelli C, Lambardi M, Benson EE (2011) Effects of osmotic pretreatments on oxidative stress, antioxidant profiles and cryopreservation of olive somatic embryos. Plant Sci 181:47–56
Martinez-Montero M, Harding K (2015) Cryobionomics: evaluating the concept in plant cryopreservation. In: Barh D, Khan MS, Davies E (eds) PlantOmics: the omics of plant science. Springer India, New Delhi, pp 655–682
Menon A, Funnekotter B, Kaczmarczyk A, Bunn E, Turner S, Mancera RL (2012) Cryopreservation of Lomandra sonderi (Asparagaceae) shoot tips using droplet-vitrification. CryoLetters 33:259–270
Menon A, Funnekotter B, Kaczmarczyk A, Bunn E, Turner S, Mancera RL (2014) Cold-induced changes affect survival after exposure to vitrification solution during cryopreservation in the south-west Australian Mediterranean climate species Lomandra sonderi (Asparagaceae). Plant Cell Tissue Organ Cult 119:347–358
Nagel M, Kranner I, Neumann K, Rolletschek H, Seal CE, Colville L, Fernández-Marín B, Börner A (2014) Genome-wide association mapping and biochemical markers reveal that seed ageing and longevity are intricately affected by genetic background and developmental and environmental conditions in barley. Plant Cell Environ. doi:10.1111/pce.12474
Prasad TK (1996) Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J 10:1017–1026
Reed BM (2008) Cryopreservation—practical considerations. In: Reed BM (ed) Plant cryopreservation. A practical guide. Springer, New York, pp 3–13
Ren L, Zhang D, Jiang X-N, Gai Y, Wang W-M, Reed BM, Shen X-H (2013) Peroxidation due to cryoprotectant treatment is a vital factor for cell survival in Arabidopsis cryopreservation. Plant Sci 212:37–47
Ren L, Zhang D, G-q Chen, Reed BM, X-h Shen, H-y Chen (2015) Transcriptomic profiling revealed the regulatory mechanism of Arabidopsis seedlings response to oxidative stress from cryopreservation. Plant Cell Rep 34:2161–2178
Roach T, Ivanova M, Beckett RP, Minibayeva FV, Green I, Pritchard HW, Kranner I (2008) An oxidative burst of superoxide in embryonic axes of recalcitrant sweet chestnut seeds as induced by excision and desiccation. Physiol Plant 133:131–139
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sánchez-Fernández R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inzé D, May MJ (1997) Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc Natl Acad Sci 94:2745–2750
Sasaki H, Ichimura K, Oda M (1996) Changes in sugar content during cold acclimation and deacclimation of cabbage seedlings. Ann Bot 78:365–369
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–1212
Skyba M, Urbanová M, Kapchina-Toteva V, Košuth J, Harding K, Čellárová E (2010) Physiological, biochemical and molecular characteristics of cryopreserved Hypericum perforatum L. shoot tips. CryoLetters 31:249–260
Skyba M, Petijová L, Košuth J, Koleva DP, Ganeva TG, Kapchina-Toteva VM, Čellárová E (2012) Oxidative stress and antioxidant response in Hypericum perforatum L. plants subjected to low temperature treatment. J Plant Physiol 169:955–964
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Ann Rev Plant Physiol 35:543–584
Thomashow MF (1998) Role of cold-responsive genes in plant freezing tolerance. Plant Physiol 118:1–7
Turner S, Touchell D, Dixon K, Tan B (2000) Cryopreservation of Anigozanthos viridis ssp. viridis and related taxa from the south-west of Western Australia. Aust J Bot 48:739–744
Uchendu EE, Leonard SW, Traber MG, Reed BM (2010a) Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cell Rep 29:25–35
Uchendu EE, Muminova M, Gupta S, Reed BM (2010b) Antioxidant and anti-stress compounds improve regrowth of cryopreserved Rubus shoot tips. In Vitro Cell Dev Biol Plant 46:386–393
Wang ZC, Deng XX (2004) Cryopreservation of shoot-tips of citrus using vitrification: effect of reduced form of glutathione. CryoLetters 25:43–50
Wen B, Wang R, Cheng H, Song S (2010) Cytological and physiological changes in orthodox maize embryos during cryopreservation. Protoplasma 239:57–67
Western Australian Herbarium (1998) FloraBase—the Western Australian flora. Department of Environment and Conservation, Kensington
Whitaker C, Beckett RP, Minibayeva FV, Kranner I (2010) Production of reactive oxygen species in excised, desiccated and cryopreserved explants of Trichilia dregeana Sond. South Afr J Bot 76:112–118
Zhang D, Ren L, G-q Chen, Zhang J, Reed BM, X-h Shen (2015) ROS-induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Rep 34:1499–1513
Acknowledgements
The authors acknowledge the Australian Research Council (Grants LP0884027 and LP140100993) and generous support from Alcoa of Australia Ltd. The Royal Botanic Gardens, Kew receives grant-in-aid from Defra, UK. BF was recipient of an Australian Postgraduate Award and MERIWA scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Emmanuel Guiderdoni.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Funnekotter, B., Colville, L., Kaczmarczyk, A. et al. Monitoring of oxidative status in three native Australian species during cold acclimation and cryopreservation. Plant Cell Rep 36, 1903–1916 (2017). https://doi.org/10.1007/s00299-017-2204-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2204-2