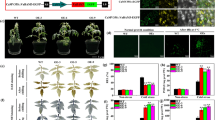
Abstract
Key message
CsHSP17.7, CsHSP18.1, and CsHSP21.8 expressions are induced by heat and cold stresses, and CsHSP overexpression confers tolerance to heat and cold stresses in transgenic Pichia pastoris and Arabidopsis thaliana.
Abstract
Small heat shock proteins (sHSPs) are crucial for protecting plants against biotic and abiotic stresses, especially heat stress. However, knowledge concerning the functions of Camellia sinensis sHSP in heat and cold stresses remains poorly understood. In this study, three C. sinensis sHSP genes (i.e., CsHSP17.7, CsHSP18.1, and CsHSP21.8) were isolated and characterized using suppression subtractive hybridization (SSH) technology. The CsHSPs expression levels in C. sinensis leaves were significantly up-regulated by heat and cold stresses. Phylogenetic analyses revealed that CsHSP17.7, CsHSP18.1, and CsHSP21.8 belong to sHSP Classes I, II, and IV, respectively. Heterologous expression of the three CsHSP genes in Pichia pastoris cells enhanced heat and cold stress tolerance. When exposed to heat and cold treatments, transgenic Arabidopsis thaliana plants overexpressing CsHSP17.7, CsHSP18.1, and CsHSP21.8 had lower malondialdehyde contents, ion leakage, higher proline contents, and transcript levels of stress-related genes (e.g., AtPOD, AtAPX1, AtP5CS2, and AtProT1) compared with the control line. In addition, improved seed germination vigor was also observed in the CsHSP-overexpressing seeds under heat stress. Taken together, our results suggest that the three identified CsHSP genes play key roles in heat and cold tolerance.






Similar content being viewed by others
Abbreviations
- DEG:
-
Differentially expressed gene
- EGFP:
-
Enhanced green fluorescent protein
- HSP:
-
Heat shock protein
- MDA:
-
Malondialdehyde
- ORF:
-
Open reading frame
- PCR:
-
Polymerase chain reaction
- RACE:
-
Rapid amplification of cDNA ends
- sHSP:
-
Small heat shock protein
- SSH:
-
Suppression subtractive hybridization
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273
Bondino HG, Valle EM, ten Have A (2012) Evolution and functional diversification of the small heat shock protein/α-crystallin family in higher plants. Planta 235:1299–1313
Charng YY, Liu HC, Liu NY, Hsu FC, Ko SS (2006) Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol 140:1297–1305
Chauhan H, Khurana N, Nijhavan A, Khurana JP, Khurana P (2012) The wheat chloroplastic small heat shock protein (sHSP26) is involved in seed maturation and germination and imparts tolerance to heat stress. Plant Cell Environ 35:1912–1931
Dejong WW, Leunissen JA, Voorter CE (1993) Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol 10:103–126
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Fragkostefanakis S, Roth S, Schleiff E, Scharf KD (2015) Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ 38:1881–1895
Gao CQ, Jiang B, Wang YC, Liu GF, Yang CP (2012) Overexpression of a heat shock protein (ThHSP18.3) from Tamarix hispida confers stress tolerance to yeast. Mol Biol Rep 39:4889–4897
Guan JC, Jinn TL, Yeh CH, Feng SP, Chen YM, Lin CY (2004) Characterization of the genomic structures and selective expression profiles of nine class I small heat shock protein genes clustered on two chromosomes in rice (Oryza sativa L.). Plant Mol Biol 56:795–809
Haslbeck M, Vierling E (2015) A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol 427:1537–1548
Huey RB et al (2002) Plants versus animals: do they deal with stress in different ways? Integr Comp Biol 42:415–423
Jiang CH, Xu JY, Zhang H, Zhang X, Shi JL, Li M, Ming F (2009) A cytosolic class I small heat shock protein, RcHSP17.8, of Rosa chinensis confers resistance to a variety of stresses to Escherichia coli, yeast and Arabidopsis thaliana. Plant Cell Environ 32:1046–1059
Kaur H et al (2015) Differentially expressed seed aging responsive heat shock protein OsHSP18.2 implicates in seed vigor, longevity and improves germination and seedling establishment under abiotic stress. Front Plant Sci 6:713
Kim KK, Kim R, Kim SH (1998) Crystal structure of a small heat-shock protein. Nature 394:595–599
Kobayashi M, Katoh H, Takayanagi T, Suzuki S (2010) Characterization of thermotolerance-related genes in grapevine (Vitis vinifera). J Plant Physiol 167:812–819
Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Kumar R, Lavania D, Singh AK, Negi M, Siddiqui MH, Al-Whaibi MH, Grover A (2015) Identification and characterization of a small heat shock protein 17.9-CII gene from faba bean (Vicia faba L.). Acta Physiol Plant 37:190
Kumar R, Kumari B, Kumar M (2016) PredHSP: sequence based proteome-wide heat shock protein prediction and classification tool to unlock the stress biology. PLoS One 11(5):e0155872
Larkin MA et al (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
Lee SH et al (2015) Identification and functional characterization of siberian wild rye (Elymus sibiricus L.) small heat shock protein 16.9 gene (EsHsp16.9) conferring diverse stress tolerance in prokaryotic cells. Biotechnol Lett 37:881–890
Li XW, Feng ZG, Yang HM, Zhu XP, Liu J, Yuan HY (2010) A novel cold-regulated gene from Camellia sinensis, CsCOR1, enhances salt- and dehydration-tolerance in tobacco. Biochem Biophys Res Commun 394:354–359
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lobell DB, Gourdji SM (2012) The influence of climate change on global crop productivity. Plant Physiol 160:1686–1697
Mu CJ et al (2011) Small heat shock protein LimHSP16.45 protects pollen mother cells and tapetal cells against extreme temperatures during late zygotene to pachytene stages of meiotic prophase I in David Lily. Plant Cell Rep 30:1981–1989
Mu CJ, Zhang SJ, Yu GZ, Chen N, Li XF, Liu H (2013) Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis enhances tolerance to abiotic stresses. PLoS One 8(12):e82264
Neta-Sharir I, Isaacson T, Lurie S, Weiss D (2005) Dual role for tomato heat shock protein 21: protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. Plant Cell 17:1829–1838
Paul A, Kumar S (2013) Dehydrin2 is a stress-inducible, whereas dehydrin1 is constitutively expressed but up-regulated gene under varied cues in tea [Camellia sinensis (L.) O. Kuntze]. Mol Biol Rep 40:3859–3863
Qin F, Sakuma Y, Li J, Liu Q, Li YQ, Shinozaki K, Yamagushi-Shinozaki KY (2004) Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol 45:1042–1052
Sabehat A, Lurie S, Weiss D (1998) Expression of small heat-shock proteins at low temperatures—a possible role in protecting against chilling injuries. Plant Physiol 117:651–658
Sahebi M, Hanafi MM, Azizi P, Hakim A, Ashkani S, Abiri R (2015) Suppression subtractive hybridization versus next-generation sequencing in plant genetic engineering: challenges and perspectives. Mol Biotechnol 57:880–903
Samanta P, Sadhukhan S, Basu A (2015) Identification of differentially expressed transcripts associated with bast fibre development in Corchorus capsularis by suppression subtractive hybridization. Planta 241:371–385
Sato Y, Yokoya S (2008) Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. Plant Cell Rep 27:329–334
Sun WN, Bernard C, van de Cotte B, Van Montagu M, Verbruggen N (2001) At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J 27:407–415
Sun WN, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. BBA Gene Struct Expr 1577:1–9
Sun X, Sun C, Li Z, Hu Q, Han L, Luo H (2016) AsHSP17, a creeping bentgrass small heat shock protein modulates plant photosynthesis and ABA-dependent and independent signalling to attenuate plant response to abiotic stress. Plant Cell Environ 39:1320–1337
Swindell WR, Huebner M, Weber AP (2007) Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom 8:125
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
van Montfort RLM, Basha E, Friedrich KL, Slingsby C, Vierling E (2001) Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol 8:1025–1030
Wang WX, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang L, Li XW, Zhao Q, Jing SL, Chen SF, Yuan HY (2009) Identification of genes induced in response to low-temperature treatment in tea leaves. Plant Mol Biol Rep 27:257–265
Wang Y, Jiang CJ, Li YY, Wei CL, Deng WW (2012) CsICE1 and CsCBF1: two transcription factors involved in cold responses in Camellia sinensis. Plant Cell Rep 31:27–34
Wang XC et al (2013) Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genom 14:415
Wang WD et al (2014) Overexpression of Camellia sinensis H1 histone gene confers abiotic stress tolerance in transgenic tobacco. Plant Cell Rep 33:1829–1841
Wang Y, Shu Z, Wang W, Jiang X, Li D, Pan J, Li X (2016) CsWRKY2, a novel WRKY gene from Camellia sinensis, is involved in cold and drought stress responses. Biol Plant 60:443–451
Waters ER (2013) The evolution, function, structure, and expression of the plant sHSPs. J Exp Bot 64:391–403
Wei K et al (2013) Identification of genes involved in indole-3-butyric acid-induced adventitious root formation in nodal cuttings of Camellia sinensis (L.) by suppression subtractive hybridization. Gene 514:91–98
Weston DJ, Karve AA, Gunter LE, Jawdy SS, Yang XH, Allen SM, Wullschleger SD (2011) Comparative physiology and transcriptional networks underlying the heat shock response in Populus trichocarpa, Arabidopsis thaliana and Glycine max. Plant Cell Environ 34:1488–1506
Wu ZJ, Tian C, Jiang Q, Li XH, Zhuang J (2016) Selection of suitable reference genes for qRT-PCR normalization during leaf development and hormonal stimuli in tea plant (Camellia sinensis). Sci Rep 6:19748
Xia XW, Gui RY, Yang HY, Fu Y, Wei F, Zhou MB (2015) Identification of genes involved in color variation of bamboo culms by suppression subtractive hybridization. Plant Physiol Biochem 97:156–164
Yao LM, Wang B, Cheng LJ, Wu TL (2013) Identification of key drought stress-related genes in the hyacinth bean. PLoS One 8(3):e58108
Zhang XR, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1:641–646
Zhang L, Gao YK, Pan HT, Hu WJ, Zhang QX (2013) Cloning and characterisation of a Primula heat shock protein gene, PfHSP17.1, which confers heat, salt and drought tolerance in transgenic Arabidopsis thaliana. Acta Physiol Plant 35:3191–3200
Zhang J et al (2015) Hsf and Hsp gene families in Populus: genome-wide identification, organization and correlated expression during development and in stress responses. BMC Genom 16:181
Zheng C et al (2015) Integrated RNA-Seq and sRNA-Seq analysis identifies chilling and freezing responsive key molecular players and pathways in tea plant (Camellia sinensis). PLoS One 10(4):e0125031
Zhou YL et al (2012) NnHSP17.5, a cytosolic class II small heat shock protein gene from Nelumbo nucifera, contributes to seed germination vigor and seedling thermotolerance in transgenic Arabidopsis. Plant Cell Rep 31:379–389
Zhu XJ, Li QH, Hu JY, Wang ML, Li XH (2015) Molecular cloning and characterization of spermine synthesis gene associated with cold tolerance in tea plant (Camellia sinensis). Appl Biochem Biotech 177:1055–1068
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31570689, 31470690) and the China Earmarked Fund for Modern Agro-industry Technology Research System (CARS-23).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Emmanuel Guiderdoni.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, M., Zou, Z., Li, Q. et al. Heterologous expression of three Camellia sinensis small heat shock protein genes confers temperature stress tolerance in yeast and Arabidopsis thaliana . Plant Cell Rep 36, 1125–1135 (2017). https://doi.org/10.1007/s00299-017-2143-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2143-y