Abstract
Key Message
This is the first study on peanut VDE, which led to multiple biochemical and physiological changes to heat and HI stress by improving de-epoxidation of the xanthophylls cycle.
Abstract
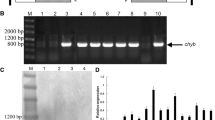
A peanut (Arachis hypogaea L.) violaxanthin de-epoxidase gene (AhVDE) was isolated by RT-PCR and RACE methods. The deduced amino acid sequence of AhVDE showed high identities with violaxanthin de-epoxidase of other plant species. The expression of AhVDE was obviously upregulated by 4, 40 °C and high light, NaCl, and abscisic acid. Sense and RNAi transgenic tobaccos were further used to investigate the physiological effects and functional mechanism of AhVDE. Compared with WT, the content of Z, the ratio of (A + Z)/(V + A + Z) and the non-photochemical quenching were higher in sense plants, and lower in the RNAi lines under heat and high irradiance (HI) stress, respectively. Additionally, photoinhibition of photosystem II (PSII) reflected by the maximal photochemical efficiency in WT lines was more severe, and in the RNAi lines was the most severe compared with that in the sense lines. Meanwhile, overexpressing AhVDE also led to multiple biochemical and physiological changes under heat and HI stress. Higher activities of superoxide dismutase and ascorbate peroxidase, lower content of reactive oxygen species and slighter membrane damage were observed in sense lines after heat and HI stress. These results suggested that, peanut VDE can alleviate PSII photoinhibition to heat and HI stress by improving the xanthophyll cycle-dependent energy dissipation.







Similar content being viewed by others
Abbreviations
- A:
-
Antheraxanthin
- ABA:
-
Abscisic acid
- APX:
-
Ascorbate peroxidase
- DAB:
-
3,3-diaminobenzidine
- Fv/Fm:
-
The maximal photochemical efficiency of PSII
- MDA:
-
Measurement of malondialdehyde
- MGDG:
-
Monogalactosyldiacylglycerol
- MS:
-
Murashige and Skoog
- NBT:
-
Nitroblue tetrazolium
- NPQ:
-
Nonphotochemical quenching
- PSII:
-
Photosystem II
- REC:
-
Relative electric conductivity
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse-transcription polymerase chain reaction
- SD:
-
Standard deviation
- SOD:
-
Superoxide dismutase
- V:
-
Violaxanthin
- VDE:
-
Violaxanthin de-epoxidase
- Z:
-
Zeaxanthin
- ZE:
-
Zeaxanthin epoxidase
References
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Baroli I, Do AD, Yamane T, Niyogi KK (2003) Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell 15:992–1008
Björkman O, Demmig-Adams B (1994) Regulation of photosynthetic light energy capture, conversion and dissipation in leaves of higher plants. In: Schulze E-D, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 17–47
Bugos RC, Hieber D, Yamamoto HY (1998) Xanthophyll cycle enzymes are members of the lipocalin family, the first identified from plants. J Biol Chem 273(25):15321–15324
Chaves M, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, Clávalo I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field. Photosynthesis and growth. Ann Bot (Lond) 89:907–916
Demmig B, Winter K, Krüger A, Czygan FC (1987) Photoinhibition and zeaxanthin formation in intact leaves. A possible role of the xanthophylls cycle in the dissipation of excess light energy. Plant Physiol 84:218–224
Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:599–626
Demmig-Adams B, Gilmore AM, Adams WW (1996) In vivo functions of carotenoids in higher plants. FASEB J 10:403–412
Di Castri F (1973) Climatographical comparisons between Chile and the western coast of North America. In: Di Castri F, Mooney HA (eds) Mediterranean-type ecosystems. Springer-Verlag, Berlin, pp 21–39
Drew A, Fleming GR, Head-Gordon M (2005) Role of electron-transfer quenching of chlorophyll fluorescence by carotenoids in non-photochemical quenching of green plants. Biochem Soc Trans 33:858–862
Gao ZM, Liu Q, Zheng B, Chen Y (2013) Molecular characterization and primary functional analysis of PeVDE, a violaxanthin de-epoxidase gene from bamboo (Phyllostachys edulis). Plant Cell Rep 32:1381–1391
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59(2):309–314
Gilmore AM (1997) Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plantarum 99:197–209
Hager A, Holocher K (1994) Localization of xanthophyll-cycle enzyme violaxanthin deepoxidase within the thylakoid lumen and abolition of its mobility by a (lightdepend) pH decrease. Planta 192:581–589
Han H, Gao S, Li B, Dong XC, Feng HL, Meng QW (2010) Overexpression of violaxanthin de-epoxidase gene alleviates photoinhibition of PSII and PSI in tomato during high light and chilling stress. J Plant Physiol 167:176–183
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96:8762–8767
Havaux M, Dall’Osto L, Cuinè S, Giuliano G, Bassi R (2004) The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J Biol Chem 279:13878–13888
Hieber AD, Bugos RC, Yamamoto Y (2000) Plant lipocalins: violaxanthin de-epoxidase and zeaxanthin epoxidase. Biochim Biophys Acta 1482:84–91
Horton P, Wentworth M, Ruban AV (2005) Control of light harvesting function of chloroplast membranes: the LHC II aggregation model for non-photochemical quenching. FEBS Lett 579:4201–4206
Hückelhoven R, Fodor J, Trujillo M, Kogel KH (2000) Barley Mla and Rar mutants compromised in the hypersensitive cell death response against Blumeria graminis f. sp. hordei are modified in their ability to accumulate reactive oxygen intermediates at sites of fungal invasion. Planta 212:16–24
Ivanov AG, Krol M, Maxwell D, Huner NP (1995) Abscisic acid induced protection against photoinhibition of PSII correlates with enhanced activity of the xanthophyll cycle. FEBS Lett 371:61–64
Jimenez A, Hernandez JA, Del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate–glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114(1):275–284
Johnson MP, Havaux M, Triantaphylidès C, Ksas B, Pascal AA, Robert B, Davison PA, Ruban AV, Horton P (2007) Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J Biol Chem 282:22605–22618
Lee AJ, Thornber JP (1995) Analysis of the pigment-protein complexes from barley (Hordeum vulgare): the xanthophyll cycle intermediates occur mainly in the light-harvesting complexes of photosystem I and II. Plant Physiol 107:565–574
Li XG, Meng QW, Jiang GQ, Zou Q (2003) The susceptibility of cucumber and sweet pepper to chilling under low irradiance is related to energy dissipation and water-water cycle. Photosynthetica 41:259–265
Li XG, Li JY, Zhao JP, Xu PL, He QW (2007) Xanthophyll cycle and inactivation of photosystem II reaction centers alleviating reducing pressure to photosystem I in morning glory leaves under short-term high irradiance. J Integr Plant Biol 49:1047–1053
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Morosinotto T, Baronio R, Bassi R (2002) Dynamics of chromophore binding to Lhc proteins in vivo and in vitro during operation of the xanthophyll cycle. J Biol Chem 277:36913–36920
Mubarakshina MM, Ivanov BN, Naydov IA, Hillier W, Badger MR, Krieger-Liszkay A (2010) Production and diffusion of chloroplastic H2O2 and its implication to signalling. J Exp Bot 61:3577–3587
Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Müller-Moulé P, Havaux M, Niyogi KK (2003) Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol 133:748–760
Neubauer C, Yamamoto HY (1992) Mehler-peroxidase reaction mediate zeaxanthin formation and zeaxanthin-related fluorescence quenching in intact chloroplasts. Plant Physiol 99:1354–1361
Nilkens M, Kress E, Lambrev PH, Miloslavina Y, Müller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of nophotochemical quenching of chlorophyll fluorescence generated under steady state conditions in Arabidopsis. Biochim Biophys Acta 1797:466–475
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Phys 50:333–359
Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10:1121–1134
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
North HM, Frey A, Boutin JP, Sotta B, Marion-Poll A (2005) Analysis of xanthophyll cycle gene expression during the adaptation of Arabidopsis to excess light and drought stress: changes in RNA steady-state levels do not contribute to shortterm responses. Plant Sci 169:115–124
Pastense C, Pimentel P, Lillo J (2005) Leaf movements and photoinhibition in relation to water stress in field-grown beans. J Exp Bot 56:425–433
Peñuelas J, Munné-Bosch S, Llusia J, Fililla I (2004) Leaf reflectance and photo- and antioxidant protection in field-grown summer-stressed Phillyrea angustifolia. Optical signals of oxidative stress? New Phytol 162:115–124
Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17:603–614
Rockholm DC, Yamamoto HY (1996) Violaxanthin de-epoxidase. Plant Physiol 110:697–703
Ruban AV, Berera R, Ilioaia C, van Stokkum IH, Kennis JT, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450:575–578
Sairam PK, Srivastava GC (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Sigrist CJA, Cerutti L, Castro E, Langendijk-Genevaux PS, Bulliard V, Bairoch A, Hulo N (2010) PROSITE, a protein domain database for functional characterization and annotation. Nucleic Acids Res 38:D161–D166
Sui N, Li M, Liu XY, Wang N, Fang W, Meng QW (2007) Response of xanthophyll cycle and chloroplastic antioxidant enzymes to chilling stress in tomato over-expressing glycerol-3-phosphate acyltransferase gene. Photosynthetica 45(3):447–454
Thayer SS, Björkman O (1992) Carotenoid distribution and deepoxidation in thylakoid pigment-protein complexes from cotton leaves and bundle-sheath cells of maize. Photosynth Res 33:213–225
Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148:960–968
Van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Verhoeven AS, Bugos RC, Yamamoto HY (2001) Transgenic tobacco with suppressed zeaxanthin formation is susceptible to stress-induced photoinhibition. Photosynth Res 67:27–39
Wang AG, Luo GH (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun 26:55–57
Wang N, Fang W, Han H, Sui N, Li B, Meng QW (2008) Overexpression of zeaxanthin epoxidase gene enhances the sensitivity of tomato PSII photoinhibition to high light and chilling stress. Physiol Plant 132:384–396
Wang N, Li B, Feng HL, Zhang QY, Yang XH, Meng QW (2010) Antisense-mediated suppression of tomato zeaxanthin epoxidase alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. Photosynthetica 48:409–416
Yamamoto HY, Higashi RM (1978) Violaxanthin de-epoxidase lipid composition and substrate specificity. Arch Biochem Biophys 190:514–522
Zhao SJ, Meng QW, Xu CC, Han HY, Zou Q (1995) Analysis of xanthophylls cycle components in plant tissues by high performance liquid chromatography. Plant Physiol Commun 31:438–442
Acknowledgments
This work was supported by the Project of Significant Application of Agricultural Technology Innovation in Shandong Province, International Science and Technology Cooperation Program of China (2015DFA31190), the Supporting Plan of National Science and Technology of China (2014BAD11B04) and the earmarked fund for Modern Agro-industry Technology Research System (CARS-14) and Shandong Major Projects of Independent Innovation Achievement Transformation (2012ZHZXIA0418).
Conflict of interest
The authors declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Peña.
Rights and permissions
About this article
Cite this article
Yang, S., Meng, DY., Hou, LL. et al. Peanut violaxanthin de-epoxidase alleviates the sensitivity of PSII photoinhibition to heat and high irradiance stress in transgenic tobacco. Plant Cell Rep 34, 1417–1428 (2015). https://doi.org/10.1007/s00299-015-1797-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1797-6