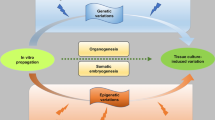
Abstract
Gradual loss of secondary metabolite production is a common obstacle in the development of a large-scale plant cell production system. In this study, cell morphology, paclitaxel (Taxol®) biosynthetic ability, and genetic and epigenetic variations in the long-term culture of Taxus media cv Hicksii cells were assessed over a 5-year period to evaluate the mechanisms of the loss of secondary metabolites biosynthesis capacity in Taxus cell. The results revealed that morphological variations, gradual loss of paclitaxel yield and decreased transcriptional level of paclitaxel biosynthesis key genes occurred during long-term subculture. Genetic and epigenetic variations in these cultures were also studied at different times during culture using amplified fragment-length polymorphism (AFLP), methylation-sensitive amplified polymorphism (MSAP), and high-performance liquid chromatography (HPLC) analyses. A total of 32 primer combinations were used in AFLP amplification, and none of the AFLP loci were found to be polymorphic, thus no major genetic rearrangements were detected in any of the tested samples. However, results from both MSAP and HPLC indicated that there was a higher level of DNA methylation in the low-paclitaxel yielding cell line after long-term culture. Based on these results, we proposed that accumulation of paclitaxel in Taxus cell cultures might be regulated by DNA methylation. To our knowledge, this is the first report of increased methylation with the prolongation of culture time in Taxus cell culture. It provides substantial clues for exploring the gradual loss of the taxol biosynthesis capacity of Taxus cell lines during long-term subculture.
Key message DNA methylation maybe involved in the regulation of paclitaxel biosynthesis in Taxus cell culture.









Similar content being viewed by others
References
Amoroso MG, Longobardo L, Capparelli R (2004) Real time RT-PCR and flow cytometry to investigate wheat kernel hardness: role of puroindoline genes and proteins. Biotechnol Lett 26:1731–1737
Bednarek PT, Orłowska R, Koebner RM, Zimny J (2007) Quantification of the tissue-culture induced variation in barley (Hordeum vulgare L.). BMC Plant Biol 7:10–18
Chan AO, Soliman AS, Zhang Q, Rashid A, Bedeir A, Houlihan PS, Mokhtar N, Al-Masri N, Ozbek U, Yaghan R, Kandilci A, Omar S, Kapran Y, Dizdaroglu F, Bondy ML, Amos CI, Issa JP, Levin B, Hamilton SR (2005) Differing DNA methylation patterns and gene mutation frequencies in colorectal carcinomas from Middle Eastern countries. Clin Cancer Res 11:8281–8287
Chen Y, Yi F, Cai M, Luo J (2003) Effects of amino acids, nitrate, and ammonium on the growth and taxol production in cell cultures of Taxus yunnanensis. Plant Growth Regul 41:265–268
Chen J, Henny RJ, Devanand PS, Chao CT (2006) AFLP analysis of nephthytis (Syngonium podophyllum Schott) selected from somaclonal variants. Plant Cell Rep 24:743–749
Croteau R, Ketchum REB, Long RM, Kaspera R, Wildung MR (2006) Taxol biosynthesis and molecular genetics. Phytochem Rev 5:75–97
Dann AL, Wilson CR (2011) Comparative assessment of genetic and epigenetic variation among regenerants of potato (Solanum tuberosum) derived from long-term nodal tissue-culture and cell selection. Plant Cell Rep 30(4):631–639
Demulemeester MA, Van Stallen N, Proft MPD (1999) Degree of DNA methylation in chicory (Cichorium intybus L.): influence of plant age and vernalization. Plant Sci 142:101–108
Georgiev MI, Weber J, Maciuk A (2009) Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biotechnol 83:809–823
Goodger JQ, Woodrow IE (2010) The influence of micropropagation on growth and coppicing ability of Eucalyptus polybractea. Tree Physiol 30:285–296
Hartweck LM, Lazzeri PA, Cui D, Collins GB, Williams EG (1988) Auxin-orientation effects on somatic embryogenesis from immature soybean cotyledons. In Vitro Cell Dev Pl 24:821–828
Hefner J, Ketchum RE, Croteau R (1998) Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase from Taxus canadensis and assessment of the role of this prenyltransferase in cells induced for taxol production. Arch Biochem Biophys 360:62–74
Hirasuna TJ, Pestchanker LJ, Srinivasan V, Shuler ML (1996) Taxol production in suspension cultures of Taxus baccata. Plant Cell Tiss Org 44:95–102
Hu FX, Huang JH, Xu YF, Gian XH, Zhong JJ (2006) Responses of defense signals, biosynthetic gene transcription and taxoid biosynthesis to elicitation by a novel synthetic jasmonate in cell cultures of Taxus chinensis. Biotechnol Bioeng 94:1064–1071
Ivan I, Peter K (2011) Origin, morphology, and anatomy of fasciation in plants cultured in vivo and in vitro. Plant Growth Regul 63(2):115–129
Jin SX, Mushke R, Zhu HG, Tu LL, Lin ZX, Zhang YX, Zhang XL (2008) Detection of somaclonal variation of cotton (Gossypium hirsutum) using cytogenetics, flow cytometry and molecular markers. Plant Cell Rep 27(8):1303–1316
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43:179–188
Kawamura M, Shigeoka T, Tahara M, Takami M, Ohashi H, Akita M, Kobayashi Y, Sakamoto T (1998) Efficient selection of cells with high taxol content from heterogeneous Taxus Cell suspensions by magnetic or fluorescent antibodies. Seibutsu Kogaku Kais 76:3–7
Kim BJ, Gibson DM, Shuler ML (2004) Effect of subculture and elicitation on instability of taxol production in Taxus sp. suspension cultures. Biotechnol Prog 20:1666–1673
Kovács P, Csaba G, Pallinger E, Czaker R (2007) Effects of taxol treatment on the microtubular system and mitochondria of Tetra hymena. Cell Biol Int 31:724–732
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Li LQ, Fu CH, Zhao CF, Xia J, Yu LJ (2009) Efficient extraction of RNA and analysis of gene expression in long-term taxus cell culture using real-time RT-PCR. Z Naturforsch C 64:125–130
Malik S, Cusidό RM, Mirjalili MH, Moyano E, Palazόn J, Bonfill M (2011) Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem 46:23–34
Morris P (1986) Long terra stability of alkaloid productivity in cell suspension cultures of Catharanthus roseus. In: Morris P, Scragg AH, Stafford A, Fowler MW (eds) Secondary metabolism in plant cell cultures. Cambridge University Press, Cambridge, pp 257–262
Neumann BD, Zenk MH (1984) Instability of indole alkaloid production in Catharanthus roseus cell suspension cultures. Planta Med 50:427–431
Pennell RI, Dang DV, Apuya N (2006) Secondary metabolite production via manipulation of genome methylation. Patent Pub. No.: US/0236421 A1
Reyna-Lόpez GE, Simpson J, Ruiz-Herrera J (1997) Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphisms. Mol Gen Genet 253:703–710
Rodríguez López CM, Wetten AC, Wilkinson MJ (2010) Progressive erosion of genetic and epigenetic variation in callus-derived cocoa (Theobroma cacao) plants. New Phytol 186:856–868
Salmon A, Clotault J, Jenczewski E, Chable V, Dauleux MJM (2008) Brassica oleracea displays a high level of DNA methylation polymorphism. Plant Sci 174:61–70
Shiio I, Ohta S (1973) Nicotine production by tobacco callus tissues and effect of plant growth regulators. Agr Biol Chem 37:1857–1864
Siemens DH, Garner SH, Mitchell-Olds T, Callaway RM (2002) Cost of defense in the context of plant competition: Brassica rapa may grow and defend. Ecology 83(2):505–517
Smulders MJM, Klerk GJD (2011) Epigenetics in plant tissue culture. Plant Growth Regul 63:137–146
Smykal P, Valledor L, Rodriguez R, Griga M (2007) Assessment of genetic and epigenetic stability in long-term in vitro shoot culture of pea (Pisum sativum L.). Plant Cell Rep 26:1985–1998
Suyama T, Ueda T, Fukasawa S, Imamura Y, Nakamura K, Miyasaka K (2009) Combination of gemcitabine and paclitaxel as second-line chemotherapy for advanced urothelial carcinoma. Jpn J Clin Oncol 39:244–250
Van der Heijden R (1988) Indole alkaloids in cell and tissue cultures of Tabernaemontana species. Ph. D. Thesis, University of Leiden, The Netherlands
Vos P, Hogers R, Bleeker M, Reijans M, Lee TVD, Hornes M (1995) AFLP: a new concept for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Walker K, Croteau R (2001) Taxol biosynthetic genes. Phytochemistry 58:1–7
Wang Y, Wu J, Yuan Y (2007) Salicylic acid-induced taxol production and isopentenyl pyrophosphate biosynthesis in suspension cultures of Taxus chinensis var. mairei. Cell Biol Int 31:1179–1183
Wani M, Taylor H, Wall M (1971) Plant antitumor agents. VI, the isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc 27:433–437
Yang C, Zhang M, Niu W, Yang R, Zhang Y, Qiu Z, Sun B, Zhao Z (2011) Analysis of DNA methylation in various swine tissues. PLoS ONE 21:e16229
Yu LJ, Lan WZ, Qin WM, Xu HB (2002) High stable production of taxol in elicited synchronous cultures of Taxus chinensis cells. Process Biochem 38:207–210
Zhang C, Fevereiro PS (2006) The effect of heat shock on paclitaxel production in Taxus yunnanensis cell suspension. Biotechnol Bioeng 96:506–514
Zhang W, Curtin C, Franco C (2002) Towards manipulation of post-biosynthetic events in secondary metabolism of plant cell cultures. Enzyme Microb Technol 30:688–696
Zhong JJ (2002) Plant cell culture for production of paclitaxel and other taxanes. J Biosci Bioeng 94:591–599
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant No.20906036 and 20776058) and Science and Technology Support Program of China (Grant No.2008BAI63B04). Thanks to Dr. Guojun Wang from Kentucky University for his revising of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Petersen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, C., Li, L., Wu, W. et al. Assessment of genetic and epigenetic variation during long-term Taxus cell culture. Plant Cell Rep 31, 1321–1331 (2012). https://doi.org/10.1007/s00299-012-1251-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1251-y