Abstract
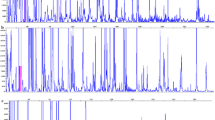
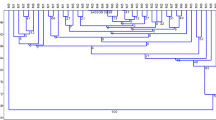
Three long-term nodal tissued cultured Russet Burbank potato clones and nine thaxtomin A-treated regenerant lines, derived from the nodal lines, were assessed for genetic and epigenetic (in the form of DNA methylation) differences by AFLP and MSAP. The treated regenerant lines were originally selected for superior resistance to common scab disease and acceptable tuber yield in pot and field trials. The long-term, tissue culture clone lines exhibited genetic (8.75–15.63% polymorphisms) and epigenetic (12.56–26.13% polymorphisms) differences between them and may represent a stress response induced by normal plant growth disruption. The thaxtomin A-treated regenerant lines exhibited much higher significant (p < 0.05) genetic (2–29.38%) and epigenetic (45.22–51.76%) polymorphisms than the nodal cultured parent clones. Methylation-sensitive mutations accumulated within the regenerant lines are significantly correlated (p < 0.05) to disease resistance. However, linking phenotypic differences that could be of benefit to potato growers, to single gene sequence polymorphisms in a tetraploid plant such as the potato would be extremely difficult since it is assumed many desirable traits are under polygenic control.





Similar content being viewed by others
References
Abou-Taleb EM, Aboshosha SM, El-Sherif EM, El-Komy MH (2010) Genetic diversity among late blight resistant and susceptible potato genotypes. Saudi J Biol Sci 17:133–138
Alvarez ME, Nota F, Cambiagno DA (2010) Review: epigenetic control of plant immunity. Mol Plant Pathol 11:563–576
Aversano R, Ercolano MR, Frusciante L, Monti L, Bradeen JM, Cristinzio G, Zoina A, Greco N, Vitale S, Carputo D (2007) Resistance traits and AFLP characterization of diploid primitive tuber-bearing potatoes. Genet Resour Crop Evol 54:1797–1806
Baránek M, Křižan B, Ondrušíková E, Pidra M (2010) DNA-methylation changes in grapevine somaclones following in vitro culture and thermotherapy. Plant Cell Tiss Organ Cult 101:11–22
Bayazit S, Kazan K, Gülbitti S, Çevik V, Ayanoğlu H, Ergül A (2007) AFLP analysis of genetic diversity in low chill requiring walnut (Juglans regia L.) genotypes from Hatay, Turkey. Sci Hortic 111:394–398
Bonin A, Pompanon F, Taberlet P (2005) Use of amplified fragment length polymorphism (AFLP) markers in surveys of vertebrate diversity. Methods Enzymol 395:145–161
Bordallo PN, Silva DH, Maria J, Cruz CD, Fontes EP (2004) Somaclonal variation on in vitro callus culture potato cultivars. Hortic Bras 22:1–6
Boyko A, Kathiria P, Zemp FJ, Yao Y, Pogribny I, Kovalchuk I (2007) Transgenerational changes in the genome stability and methylation in pathogen-infected plants: (Virus-induced plant genome instability). Nucleic Acids Res 35:1714–1725
Cassells AC (1998) In vitro-induced mutations for disease resistance. In: Mohan Jain S, Brar DS, Ahloowalia BS (eds) Somaclonal variation and induced mutations in crop improvement. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 367–379
de la Puente R, González AI, Ruiz ML, Polanco C (2008) Somaclonal variation in rye (Secale cereale L.) analysed using polymorphic and sequenced AFLP markers. In Vitro Cell Develop Biol Plant 44:419–426
Duchesne P, Bernatchez L (2002) AFLPOP: a computer program for simulated and real population allocation based on AFLP data. Mol Ecol Notes 3:380–383
Fry BA, Loria R (2002) Thaxtomin A: evidence for a plant cell wall target. Physiol Mol Plant Pathol 60:1–8
Goth RW, Haynes KG, Young RJ, Wilson DR, Lauer FI (1985) Relative resistance of the potato cultivar Krantz to common scab caused by Streptomyces scabies as determined by cluster analysis. Am J Potato Res 72:505–511
Grant-Downton RT, Dickinson HG (2005) Epigenetics and its implications for plant biology. 1. The epigenetic network in plants. Ann Bot 96:1143–1164
Guo WL, Wu R, Zhang YF, Liu XM, Wang HY, Gong L, Zhang ZH, Liu B (2007) Tissue culture-induced locus-specific alteration in DNA methylation and its correlation with genetic variation in Codonopsis lanceolata Benth et Hook. f. Plant Cell Rep 26:1297–1307
Guthridge KM, Dupal MP, Kölliker R, Jones ES, Smith KF, Forster JW (2001) AFLP analysis of genetic diversity within and between populations of perennial ryegrass (Lolium perenne L.). Euphytica 122:191–201
Hao Y-J, Wen X-P, Deng X-X (2004) Genetic and epigenetic evaluations of citrus calluses recovered from slow-growth culture. J Plant Physiol 161:479–484
Hegarty MJ, Hiscock SJ (2008) Genomic clues to the evolutionary success of polyploid plants. Curr Biol 18:R435–R444
Joyce SM, Cassells AC (2002) Variation in potato microplant morphology in vitro and DNA methylation. Plant Cell Tissue Organ Cult 70:125–137
Kuznetsova OI, Ash OA, Gostimsky SA (2006) The effect of the duration of callus culture on the accumulation of genetic alterations in pea Pisum sativum L. Russ J Genet 42:555–562
Lakshmanan V, Venkataramareddy SR, Neelwarne B (2007) Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron J Biotechnol 10:1–8
Laurentin H, Karlovsky P (2007) AFLP fingerprinting of sesame (Sesamum indicum L.) cultivars: identification, genetic relationship and comparison of AFLP informativeness parameters. Genet Resour Crop Evol 54:1437–1446
Li X, Yu X, Wang N, Feng Q, Dong Z, Liu L, Shen J, Liu B (2007) Genetic and epigenetic instabilities induced by tissue culture in wild barley (Hordeum brevisubulatum (Trin.) link). Plant Cell Tissue Organ Cult 90:153–168
Loria R, Bukhalid RA, Fry BA, King RR (1997) Plant pathogenicity in the genus Streptomyces. Plant Dis 81:836–846
Lucht JM, Mauch-Mani B, Steiner H-K, Metraux J-P, Ryals J, Hohn B (2002) Pathogen stress increases somatic recombination frequency in Arabidopsis. Nat Genet 30:311
Mantel NA (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Matthes M, Singh R, Cheah S-C, Karp A (2001) Variation in oil palm (Elaeis guineessis Jacq.) tissue culture-derived regenerants revealed by AFLPs with methylation-sensitive enzymes. Theor Appl Genet 120:971–979
McClelland M, Nelson M, Raschke E (1994) Effect of site-specific modification on restriction endonucleases and DNA modification methyltransferases. Nucleic Acids Res 22:3640–3659
McGregor CE, Lambert CA, Greyling MM, Louw JH, Warnich L (2000) A comparative assessment of DNA fingerprinting techniques (RAPD, ISSR AFLP and SSR) in tetraploid potato (Solanum tuberosum L.) germplasm. Euphytica 113:135–144
Nielsen SL, Bång H, Kotkas K, Kristensen K, Palohuhta JP, Rosenberg V, Tolstrup K (2007) Variation of growth and disease characters between clones of potato (Solanum tuberosum L.). Potato Res 50:97–114
Pacheco G, Cardoso SRS, Gagliardi RF, Valls JFM, Ferreira PCG, Cardoso MA, Mansur E (2008) Genetic and epigenetic analyses of in vitro-grown plants of Arachis villosulicarpa Hoehne (Leguminosae) obtained from seed explants through different regeneration pathways. J Hortic Sci Biotechnol 83:737–742
Pavet V, Quintero C, Cecchini NM, Rosa AL, Alvarez ME (2006) Arabidopsis displays centromeric DNA hypomethylation and cytological alterations of heterochromatin upon attack by Pseudomonas syringae. Mol Plant Microbe Interact 19:577–587
Peredo EL, Revilla MA, Arroyo-García R (2006) Assessment of genetic and epigenetic variation in hop plants regenerated from sequential subcultures of organogenic calli. J Plant Physiol 163:1071–1079
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci 91:5222–5226
Richards EJ (2006) Inherited epigenetic variation-revisiting soft inheritance. Nat Rev Genet 7:395–401
Rodríguez-López CM, Wetten AC, Wilkinson MJ (2010) Progressive erosion of genetic and epigenetic variation in callus-derived cocoa (Theobroma cacao) plants. New Phytol 186:856–868
Saze H (2008) Epigenetic memory transmission through mitosis and meiosis in plants. Semin Cell Develop Biol 19:527–536
Schellenbaum P, Mohler V, Wenzel G, Walter B (2008) Variation in DNA methylation patterns of grapevine somaclones (Vitis vinifera L.). BMC Plant Biol 8:78–88
Sha AH, Lin XH, Huang JB, Zhang DP (2005) Analysis of DNA methylation related to rice adult plant resistance to bacterial blight based on methylation-sensitive AFLP (MSAP) analysis. Mol Gen Genomics 273:484–490
Sharma SK, Bryan GJ, Winfield MO, Millam S (2007) Stability of potato (Solanum tuberosum L.) plants regenerated via somatic embryos, axillary bud proliferated shoots, microtubers and true potato seeds: a comparative phenotypic, cytogenetic and molecular assessment. Planta 226:1449–1458
Stansfield WD (2006) Luther Burbank: honorary member of the American breeders’ association. J Hered 97:95–99
Vazquez AM (2001) Insight into somaclonal variation. Plant Biosyst 135:57–62
Vazquez AM, Linacero R (2010) Stress and somaclonal variation. In: Pua E-C, Davey MR (eds) Plant developmental biology: biotechnological perspectives. Springer, Heidelberg, Germany
Visser RGF, Bachem CWB, de Boer JM, Bryan GJ, Chakrabati SK, Feingold S, Gromadka R, van Ham RCHJ, Huang S, Jacobs JME, Kuznetsov B, de Melo PE, Milbourne D, Orjeda G, Sagredo B, Tang X (2009) Sequencing the potato genome: outline and first results to come from the elucidation of the sequence of the world’s third most important food crop. Am J Potato Res 86:417–429
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wada Y, Miyamoto K, Kusano T, Sano H (2004) Association between up-regulation of stress-responsive genes and hypomethylation of genomic DNA in tobacco plants. Mol Gen Genomics 271:658–666
Wilson CR (2004) A summary of common scab disease of potato research from Australia. In: Naito S, Kondo N, Akino S, Ogoshi A, Tanaka F (eds) International potato scab symposium 2004. Hokkaido University, Japan
Wilson CR, Ransom LM, Pemberton BM (1999) The relative importance of seed-borne inoculum to common scab disease of potato and the efficacy of seed tuber and soil treatments for disease control. J Phytopathol 147:13–18
Wilson CR, Tegg RS, Wilson AJ, Luckman GA, Eyles A, Yuan ZQ, Hingston LH, Conner AJ (2010) Stable and extreme resistance to common scab of potato obtained through somatic cell selection. Phytopathology 100:460–467
Xu M, Li X, Korban SS (2000) AFLP-based detection of DNA methylation. Plant Mol Biol Rep 18:361–368
Zilberman D, Henikoff S (2007) Genome-wide analysis of DNA methylation patterns. Development 134:3959–3965
Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S (2008) Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 456:125–129
Acknowledgments
The authors would like to thank Annabel Wilson for tissue culture and maintenance of all the potato lines, Dr. Robert Tegg for data and experimental information on field and pot trials, Peter Molesworth for help with statistical analysis and Adam Smolenski of the University of Tasmania, Central Science Laboratory for organising samples to be processed on the Beckman Coulter CEQ8000. This project had partial funding from an internal University of Tasmania grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Judelson.
Rights and permissions
About this article
Cite this article
Dann, A.L., Wilson, C.R. Comparative assessment of genetic and epigenetic variation among regenerants of potato (Solanum tuberosum) derived from long-term nodal tissue-culture and cell selection. Plant Cell Rep 30, 631–639 (2011). https://doi.org/10.1007/s00299-010-0983-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0983-9