Abstract
The increasing adoption of real-world studies in healthcare for decision making and planning has further necessitated the need for a specific quality assessment tool for evidence synthesis. This study aimed to develop a quality assessment tool for systematic reviews (SR) and meta-analysis (MA) involving real-world studies (QATSM-RWS) using a formal consensus method. Based on scoping review, the authors identified a list of items for possible inclusion in the quality assessment tool. A Delphi survey was formulated based on the identified items. A total of 89 experts, purposively recruited, with research experience in real-world data were invited to participate in the first round of Delphi survey. The participants who responded in the first Delphi round were invited to participate (n = 15) in the phrasing of the items. Strong level of agreement was found on the proposed list of items after the first round of Delphi. A rate of agreement ≥ 0.70 was used to define which items to keep in the tool. A list of 14 items emerged as suitable for QATSM-RWS. The items were structured under five domains: introduction, methods, results, discussions, and others. All participants agreed with the proposed phrasing of the items. This is the first study that has developed a specific tool that can be used to appraise the quality of SR and MA involving real-world studies. QATSM-RWS may be used by policymakers, clinicians, and practitioners when evaluating and generating real-world evidence. This tool is now undergoing validation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of real-world studies in regulatory, drug development, and healthcare is becoming increasingly important for decision making and planning [1]. Real-world studies are studies generated from real-world data which are related to patient health status or delivery of health care routinely collected from a variety of sources including the internet, social media, wearable devices, and mobile devices, claims and billing activities, registries, electronic health records (EHRs), product and disease registries, and e-health services [2]. The rising costs of traditional clinical trials and the fast development of artificial intelligence and machine learning techniques have prompted the interest in the use of real-world data [3, 4]. Real-world studies help provide an overall picture of safety of any medical product (adverse effects) that randomised control trials by themselves do not provide [5]. Real-world studies support randomised control trials, in the case of rare diseases, when recruiting suitable patients is difficult [6].
The aim of conducting real-world studies is to improve healthcare delivery and health outcomes for patients. Systematic reviews and clinical guidelines of these type of studies bring together trustworthy information and transferring this information into clinical, management, and policy arenas [7]. Several quality assessment tools have previously been developed to improve the quality of reporting of systematic reviews and meta-analysis of studies based on randomised trials and observational studies [8,9,10,11,12,13]. These quality assessment tools are useful to evaluate studies with a non-biased approach for a given topic [14]. When reading any type of evidence, being critical of all aspects of the study design, execution and reporting is vital before being applied to practice.
Increasing research studies and the precise nature of scientific discoveries may highlight the importance of developing quality assessment tools. The quality checklist of a given assessment tools may not always suit as included items may not be relevant for the purposes of the intended SR and MA [15, 16]. Thus, individual real- worlds studies included in SRs and MA should be assessed in terms of quality of reporting or potential risk of bias using its own specific assessment tool [17]. A formal quality assessment of the individual studies included in SRs and MA of real-world studies is important to evaluate the overall quality of their results. A scoping review on quality assessment tools used in SRs and MA of real-world studies identified no validated and reliable tool that are specific to these types of study [18].
Due to their unique design features, the criteria needed to assess the quality of real-world studies may differ from other studies. The adoption of a standardised QATSM-RWS may also avoid the use of different types of tools that could be a source of bias. Although several types of quality assessment tools are currently used [18], none of these have been systematically developed to evaluate SRs and MA involving real-world studies. This study was aimed to develop QATSM-RWS using a formal consensus method.
Methods
Study design
This study was approved by the Health and Education Research Ethics and Governance Committee at The Manchester Metropolitan University (EthOS Reference Number: 56368). From an initial scoping review [18], the authors identified 16 quality assessment tools that were used to assess the quality of systematic reviews and meta-analyses involving real-world studies. Using Excel spreadsheet, the list of items used by the 16 quality assessment tools were listed and helped to develop themes. Those items used as a criteria of quality assessment by more than 50% of the included studies were selected for inclusion in the proposed quality assessment tool (Table 1). The authors were responsible for item generation of the initial checklist, whereas a Delphi group of experts adapted and approved the final checklist. Consensus on eligible professionals was based on track-record and evidence of publication of real-world studies. To minimise bias, all researchers were blinded to the experts’ identities that participated to the Delphi survey. All questionnaires were developed and facilitated using the online survey platform.
The Delphi method was used to achieve expert consensus on the content of QATSM-RWS. Delphi method is useful to obtain consensus on an important topic using a structured multi-staged survey involving professionals in the area [19].
Participants
There are no universally agreed criteria for the selection of experts for a Delphi study [20]. The selection of participants was professionals from various locations. One of the authors (TG) identified participants who published studies that have used real-world data. Participants were included if they hold a postgraduate qualification, recognised through publication of real-world studies, and taught at university level. On the other hand, professionals who did not have expertise in real-world studies were excluded. Informed consent was obtained from each participant at the start of the survey, by providing participant information and requesting that participants indicate consent by clicking on the consent box. Participation was voluntary and was kept confidential throughout the study.
Study procedures
We employed a purposive sampling strategy. An invitation email was sent to the individuals who published studies using real-world data informing them of the purpose and details of the study. Their emails were obtained from the contact details of the published article. Agreeing to participate entailed clicking on the survey link to initiate the first round. The Delphi group size depends more on group dynamics in reaching consensus among experts, we aimed for the recommended minimum sample size of 15 [21]. To form a representative international expert, we included professionals from various countries.
In Delphi survey round one, the participants (n = 89) were asked to rate each item on a four-point rating scale (1 = strongly disagree, 2 = disagree, 3 = agree, 4 = strongly agree). Comment boxes were provided, allowing the participants to provide recommendations regarding any additions and/or deletions to the list of proposed items. Each survey required not more than 10 min to complete. For a Delphi study, a universally agreed minimum level of consensus does not exist but typically ranges from 50 to 80% [22]. In this study, consensus was defined a priori to include items that had a mean score of ≥ 3.5 and were rated agreed (3) or strongly agreed (4) or by ≥ 70% of the participants. Items that did not meet an agreement of ‘agreed’ or ‘strongly agreed’ for at least 70% of the participants were considered for the next round.
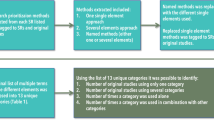
In round two, the participants (n = 15) who responded in the first Delphi round were invited to participate. The findings of the first round were reported to the participants and were asked to ‘agree’, ‘’not agree’’ with the proposed phrasing of the included items. If the participants did not agree with the phrasing, they were asked to suggest alternative phrasing or comments rather than ranking the question like the first round. The flowchart of the Delphi process is shown in Fig. 1.
Statistical analysis
Descriptive analysis was performed by calculating the mean scores, standard deviation (SD) and percentage for each item. All data analysis was done using the statistical software SPSS Statistics version 24.0 (IBM). Greater importance was associated with higher means and lower SDs.
Results
The items/domains used for quality assessment by most of published SR and MA involving real-world data were considered in this study. Following the scoping review, the authors developed a survey questionnaire that consisting of 14 questions. Based on the consensus criteria, all the included items have reached a sufficiently high level of consensus amongst all the respondents and were included in the quality assessment tool (Table 1 or Fig. 2). High levels of consensus were demonstrated, with one item: inclusion of research questions/objectives reaching 100% agreement of ‘strongly agreed’. The remaining items reaching ≥ 70% consensus agreement by the participants (n = 15).
In Delphi round 2, out of the 15 professionals who participated in round 1 Delphi survey, only 12 of them responded. All these participants agreed with the proposed phrasing of the included items (Table 2). However, some comments were proposed regarding the phrasing of the items. The authors considered all the comments from the participants carefully and made relevant changes. Thus, the final tool had face and content validity as judged by the consensus panel.
Discussion
This study aimed to develop QATSM-RWS using a formal consensus method. To our knowledge, this is the first study to report QATSM-RWS developed through the Delphi method. Analysis of real-world data are needed to generate real-world evidence [23]. According to Framework for FDA's Real-World Evidence Program [24], real-world evidence is the clinical evidence about the usage and potential benefits or risks of a medical product [25,26,27,28,29]. However, concern relating to poor data quality due to unstructured data and resulting bias have been highlighted in using real-world data to generate evidence [25,26,27]. In addition, using non-specific tools to appraise quality from real-world data may further complicate evidence generated, hence the need for a specific tool for the purpose. This study adopted the Delphi approach, accordingly 15 authors in the field of real-world studies across various backgrounds and nationalities participated in the development of the tool. The main purposes of using experts in Delphi is to increase the qualitative strength of consensus [28]. Although, there is agreement in literature on the sample size required for the panel, a range between 10–100 are common in literature. As it is the case in this study, having appropriate panel size of experts who are knowledgeable in the topic area, with varied practice specialties, academic and geographical backgrounds in the process improves the generalizability of Delphi results [28, 29]. The findings of the current study revealed a high level of consensus during the first and second round among the participants about the list of items and phrasing of the quality assessment tool.
The findings of a preliminary scoping review by Gebrye et al. [18] suggested that the generic quality assessment tools are mostly used in systematic reviews and meta-analysis involving real-world studies, while no validated and reliable specific tool currently exist. A related quality assessment tool for real-world studies by Wylde et al. [30] seems to suggest that each individual item rating is reported, rather than an overall score and it is non-summative. Hence, a Delphi survey was conducted following rigorous methodical process to develop a specific quality assessment tool with face and content validity, which also has both items and an overall quality scores. Overall quality scores is the summation of each items from a quality assessment tool [17]. Despite debates regarding weighting of individual items to provide an overall quality score [17], it continues to be adopted and used as part of the quality assessment process in systematic reviews [31, 32].
Real-world data is important to produce real-world evidence that helps to answer questions that may not be addressed by clinical trials and lab-based experiments [2]. To improve the quality and transparency of evidence it is necessary that real-world evidence studies are clearly planned, conducted, and reported. Real-world evidence has the potential to significantly improve the efficiency of health-related research and decision making. Parallel to this, systematic reviews have become the standard approach in assessing and summarizing health-related research [33]. In order to generate unbiased results from systematic reviews it is important that the quality of the included studies is assessed. The authors strongly believe that the quality assessment tool developed in the current study could be used to ensure a more focussed and coordinated approach to research in the area.
This study has some strengths and limitations that needs careful consideration during its applications. The strength of this study was the spread of experts across different continents. The authors are comfortable with the rigorous methodology used to the Delphi study making it a key strength of the current study. On the other hand, the findings reported here should be viewed in the context of the limitations of this study. This quality assessment tool was developed based on expert consensus without empirical evidence for potential sources of bias in systematic reviews and meta-analysis involving real-world studies. This study used email contacts, and some authors in real-world data might have been excluded from taking part in the study. Thus, for completeness, the developed tool needs further testing in real-world studies to ensure its applicability in a realistic setting [34].
Conclusion
A specific tool (QATSM-RWS) that can be used for quality assessment of SR and MA involving real-world studies was developed. QATSM-RWS appears to be detailed and it is easy to use. The significance of the current study is that the newly developed QATSM-RWS has implications for research, education, and practice as it may help facilitate decision making to improve access to healthcare interventions to enhance health outcomes for patients. Thus, QATSM-RWS may be used by policymakers, clinicians, and practitioners when evaluating and generating real-world evidence. This tool is now undergoing validation process.
Data availability
Data are available upon reasonable request from the authors.
References
Miksad RA, Abernethy AP (2018) Harnessing the power of real-world evidence (RWE): a checklist to ensure regulatory-grade data quality. Clin Pharmacol Ther 103(2):202–205
Liu F, Panagiotakos D (2022) Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Med Res Methodol 22(1):287
Powell AA, Power L, Westrop S, McOwat K, Campbell H, Simmons R, Amirthalingam G (2021) Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March–June 2021, England. Eurosurveillance 26(28):2100634
Hunter PR, Brainard J (2021) Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose. A reanalysis of a study of ‘real-world’ vaccination outcomes from Israel. Medrxiv, 2021–02.
Dang A, Jagan MVRP, Kishore R, Vallish BN (2021) Real world safety of bevacizumab in cancer patients: a systematic literature review of case reports. Int J Risk Saf Med 32(3):163–173
Li G, Sajobi TT, Menon BK, Korngut L, Lowerison M, James M, Thabane L (2016) Registry-based randomized controlled trials-what are the advantages, challenges, and areas for future research? J Clin Epidemiol 80:16–24
Manchikanti L (2008) Evidence-based medicine, systematic reviews, and guidelines in interventional pain management, part I: introduction and general considerations. Pain Physician 11(2):161
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet 354(9193):1896–1900
Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, European Carotid Surgery Trial Collaborative Group (2003) Evaluating non-randomised intervention studies. Health Technol Assess (Winchester, England) 7(27):iii–173
West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, Lux L (2002) Systems to rate the strength of scientific evidence: summary. In AHRQ evidence report summaries. Agency for Healthcare Research and Quality (US)
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283(15):2008–2012
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457
Altman D, Egger M, Pocock S, Vandenbrouke JP, von Elm E (2005) Strengthening the reporting of observational epidemiological studies. STROBE statement: Checklist of Essential items Version, 3
Oxman AD, Schünemann HJ, Fretheim A (2006) Improving the use of research evidence in guideline development: 8. Synthesis and presentation of evidence. Health Res Pol Syst 4(1):1–10
Brouwers MC, Johnston ME, Charette ML, Hanna SE, Jadad AR, Browman GP (2005) Evaluating the role of quality assessment of primary studies in systematic reviews of cancer practice guidelines. BMC Med Res Methodol 5:1–9
Shamliyan T, Kane RL, Jansen S (2010) Peer reviewed: quality of systematic reviews of observational nontherapeutic studies. Prevent Chronic Dis 7(6):A133
Whiting P, Harbord R, Kleijnen J (2005) No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol 5(1):1–9
Gebrye T, Fatoye F, Mbada C, Hakimi Z (2023) A scoping review on quality assessment tools used in systematic reviews and meta-analysis of real-world studies. Rheumatology Int 43:1573–1581
Keeney S, McKenna HA, Hasson F (2011) The Delphi technique in nursing and health research. Wiley
Simpson PL, Settumba S, Adily A, Ton B, Butler T (2021) Defining optimal post-prison care for those with psychosis: a Delphi study. Front Psych 12:760904
Okoli C, Pawlowski SD (2004) The Delphi method as a research tool: an example, design considerations and applications. Inform Manage 42(1):15–29
Hasson F, Keeney S, McKenna H (2000) Research guidelines for the Delphi survey technique. J Adv Nurs 32(4):1008–1015
NICE real-world evidence framework Corporate document [ECD9] Published (2022) https://www.nice.org.uk/corporate/ecd9/chapter/introduction-to-real-world-evidence-in-nice-decision-making
Framework for FDA's Real-World Evidence Program (2018) Food and Drugs Administration, US
Rudrapatna VA, Butte AJ (2020) Opportunities and challenges in using real-world data for health care. J Clin Investig 130(2):565–574
Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS (2018) Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther 35:1763–1774
Chodankar D (2021) Introduction to real-world evidence studies. Perspect Clin Res 12(3):171
Nasa P, Jain R, Juneja D (2021) Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol 11(4):116
Santaguida P, Dolovich L, Oliver D, Lamarche L, Gilsing A, Griffith LE, Raina P (2018) Protocol for a Delphi consensus exercise to identify a core set of criteria for selecting health related outcome measures (HROM) to be used in primary health care. BMC Fam Pract 19(1):1–14
Wylde V, Beswick AD, Dennis J, Gooberman-Hill R (2017) Post-operative patient-related risk factors for chronic pain after total knee replacement: a systematic review. BMJ Open 7(11):e018105
Whiting P, Rutjes AW, Dinnes J, Reitsma JB, Bossuyt PM, Kleijnen J (2004) A systematic review finds that diagnostic reviews fail to incorporate quality despite available tools. J Clin Epidemiol 58(1):1–12
Whiting P, Rutjes AW, Dinnes J, Reitsma JB, Bossuyt P M, Kleijnen J (2004) Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess 8:1–234
Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, Boers M (2009) AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 62(10):1013–1020
Brouwers MC, Spithoff K, Kerkvliet K, Alonso-Coello P, Burgers J, Cluzeau F, Florez ID (2020) Development and validation of a tool to assess the quality of clinical practice guideline recommendations. JAMA Netw Open 3(5):e205535–e205535
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TG, CEM, ZH & FF contributed to the study design, data collection, statistical analysis, interpretation of results, and drafting the manuscript. All authors had conceived, designed, analysed the data, and interpreted the results of the work. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Ethical approval
This study has been approved by the Health and Education Research Ethics and Governance Committee at The Manchester Metropolitan University (EthOS Reference Number: 56368).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gebrye, T., Mbada, C., Hakimi, Z. et al. Development of quality assessment tool for systematic reviews and meta-analyses of real-world studies: a Delphi consensus survey. Rheumatol Int 44, 1275–1281 (2024). https://doi.org/10.1007/s00296-024-05595-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-024-05595-4