Abstract
This study aimed to investigate the disease characteristics of familial Mediterranean fever (FMF) patients undergoing dose optimisation and discontinuation of canakinumab therapy. A total of 61 patients diagnosed with FMF and using canakinumab for the resistant disease were enrolled on this retrospective study. Patients’ characteristics, disease activity, treatment response, dose optimisation, dose intervals, attack-free periods, drug-free periods and side effects were noted. Dose intervals were extended in patients who achieved remission without being bound by any protocol at the discretion of the rheumatology physician who followed up with the patients in the outpatient clinic. The drug was discontinued in some patients whose dose intervals were 2 months or longer and remained in remission for 6 months or longer. A total of 57 patients (56% female, median age 32.4 years) were included. The mean attack frequency before canakinumab was 3.4/6 months, while it was 1.2 at the last post-treatment visit (p < 0.001). The median duration of canakinumab use was 46 months. After the first 6 months, the dosing interval was extended in 22 patients, and then treatment was discontinued in 12 of them who did not have an attack in the last 6 months. Three of the 12 patients whose treatment was discontinued started monthly treatment again after their attacks recurred. In the remaining ten patients, dose intervals were extended to 8–12 weeks after 6 months of monthly treatment. Nine patients are still being followed up without attacks and receive only colchicine therapy. Canakinumab is a safe and effective treatment, dose intervals may be extended, and follow-up without medication may be possible for eligible patients. However, there is a need for a consensus on dose optimisation or tapering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial Mediterranean fever (FMF) is the most common inherited autoinflammatory disease characterized by self-limiting episodes of fever, serositis, or synovitis. FMF is common among Eastern Mediterranean populations, mainly Turkish, Jewish, Armenian, and Arab; however, it can also be seen sporadically in different parts of the world [1, 2]. In 60–90% of patients with FMF, symptoms begin before the age of 20 and in 10–20% after the age of 20 [3]. The main clinical feature of the disease is the entirely normal clinical and laboratory response during attack-free periods and the acute-phase response, which increases significantly during attacks. It has been reported that acute-phase responses remain high in patients resistant to treatment in attacks-free periods. The most serious complication of the disease is amyloidosis and related end-stage renal disease [4, 5].
FMF results from the mutations of the MEFV gene that codes pyrin, a regulatory protein [6, 7]. It has been determined that mutations occur most commonly in the exon 10. Four of the five most common mutations occur in exon 10 (M694V, V726A, M694I, M680I), and one in exon 2 (E148Q). According to the genotype, homozygous M694V (24%) was the most common mutation, followed by heterozygous M694V in Turkish MFF patients [8]. Mutations disrupt pyrin regulation, resulting in caspase-1 activation, which mediates the release of interleukin-1ß (IL-1β) from its inactive precursor. The mutation of the MEFV gene in FMF stimulates IL-1β production and suppresses apoptosis, causing inflammatory attacks [9, 10].
Colchicine is the mainstay of treatment as it reduces the frequency, duration, and severity of attacks and prevents amyloidosis, but 5–10% of patients are resistant to colchicine therapy. In addition, colchicine cannot be used in effective doses in approximately 20% of patients due to its side effects [11, 12]. The discovery of the critical role of IL-1ß in the pathogenesis of FMF has led to advances in treating the disease. Today, therapeutic blockade of IL-1β cytokine is accepted as an alternative treatment regimen for patients with colchicine intolerance or inadequate response or disease complications [13]. Two agents that block IL-1β, anakinra (recombinant antagonist of the IL-1 receptor) and canakinumab (fully human monoclonal antibody against IL-1β), are used in treating FMF. From the EULAR recommendations, it is emphasized considering an anti-interleukin 1 (IL-1) therapy when inflammation cannot be controlled with the maximum tolerable colchicine dose [14, 15]. Both drugs have advantages and disadvantages regarding application frequency, effectiveness, safety and costs [16]. There is no consensus on the optimal dose and dosing interval, treatment tapering, duration of treatment, and discontinuation of the drug in adult patients who have benefited from canakinumab and are in remission. This is the first study to include long-term data on adult FMF patients on this subject. The study aims to investigate the disease characteristics, disease follow-up periods, drug use durations, and demographic characteristics of the FMF patients whose treatment dose was optimized (dose was increased, dose interval was extended, or the drug was discontinued due to remission) as well as the effectiveness and safety of the drug under routine outpatient clinic conditions.
Methods
Patients
A total of 61 patients over 18 who were followed up regularly in the rheumatology outpatient clinics between 2015 and 2021, diagnosed with FMF according to the Tel HaShomer criteria, and treated with canakinumab for 6 months or longer were included in this retrospective multicenter study. A total of 1254 patients followed for FMF were screened, and 61 patients using canakinumab were included in the study. The patients who did not attend regular control visits and did not use canakinumab regularly were excluded from the study. Four patients were excluded from the study due to lack of data and sporadic use of the drugs. The study was conducted in the routine outpatient setting by examining FMF patients' files and electronic records at each visit. These patients are followed up with detailed forms every 3 months as they receive biologic therapy. Consent was obtained from all patients before IL-1 treatment. Ethics committee approval was obtained from Ankara State Hospital Ethics Committee (dated 17.08.2022, decision number: E2-22-2259).
Data collection
Patients' demographic characteristics, clinical and laboratory findings, 24-h protein counts in urine, attack frequency, attack duration, disease duration, follow-up duration, family history, MEFV mutation, drugs used, drug doses, drug use durations, treatment response, treatment dose optimisation (dose increased, dose interval extended or discontinued), dose intervals, attack-free periods, drug-free periods and drug side effects were noted. Changes in the treatment of the patients at each follow-up visit, changes at months 1, 3, 6, 9, 12, 15, 18, and 24 of treatment, and the last visit were recorded.
The Pras score was used to assess disease activity. The Pras scoring was based on the age of disease onset, frequency of attacks, colchicine dose administered to control the attacks, joint involvement, erysipelas-like erythema, and presence of amyloidosis. 2–5 points were determined as a mild disease, 6–10 points as moderate, and ≥ 10 points as severe [17]. Colchicine treatment was continued with canakinumab in all patients. All patients were examined for tuberculosis with PPD or quantiferon test before treatment, and patients with PPD above 5 mm or positive quantiferon test were administered TB prophylaxis.
Treatment and dosing
All of the patients had previously received colchicine treatment and were switched to anakinra/canakinumab treatment as they had an inadequate response to the treatment. A patient with three or more attacks in the last 6 months was considered a colchicine-resistant patient [18]. Of the patients who received anakinra therapy, those who could not use anakinra due to injection site reaction, local or systemic allergic reaction, or non-compliance with daily use, or those who had an inadequate response to anakinra were switched to canakinumab therapy. All patients on canakinumab started 150 mg/4 weeks dose. The absence of an attack in the last 6 months and the lack of subclinical inflammation findings in the attack-free period (normal acute-phase reactants) was considered a complete response to canakinumab treatment. Partial response was defined as a decrease in the severity and rate of attacks by more than 50% with anti-IL-1 treatment and/or an increase in acute-phase reactants despite the absence of attack in patients under treatment. Persistent inflammation was considered as CRP remaining higher than normal for two weeks or longer after the last attack in the period between attacks [19]. Drug survival was defined as the time from initiation to discontinuation of canakinumab therapy.
Dose intervals were extended in patients who achieved complete response (remission) without being bound by any protocol at the discretion of the rheumatology physician who followed up with the patients in the outpatient clinic. The drug was discontinued in some patients whose dose intervals were 2 months or longer and remained in remission for 6 months or longer.
Statistical analyses
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) for Windows version 15.0 (SPSS Inc., Chicago, IL, USA). The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilk’s test) to determine whether they were normally distributed. Continuous variables were expressed as mean ± standard deviation (SD) or median (range) and categorical variables as numbers (n) and percentages (%). Friedman tests were conducted to determine whether there was a significant change in the frequency and duration of attacks and inflammatory marker levels due to violations of parametric test assumptions (non-normal distribution and a low number of cases). A value of p < 0.05 was considered statistically significant.
Results
Study population characteristics
A total of 57 patients using canakinumab for the resistant disease were included in the study. The median age was 32.4 years, and 32 patients were female. The median disease duration was 11 years (range 2–22), and the time between the onset of symptoms and diagnosis was 1.9 years. Eight patients had a consanguineous marriage, 14 had a family history of FMF, and 10 had first-degree relatives with FMF. Abdominal pain (100%), fever (93.3%), chest pain (66.7%), and arthralgia/arthritis (54%) were the most common findings. The most common mutation was M694V/M694V, detected in 40 of 57 patients. Patients' demographics and characteristics are presented in Table 1.
All patients used the maximum tolerated dose of colchicine and were resistant or intolerant to colchicine. A total of 15 patients were switched due to colchicine-related side effects and 42 patients were switched due to intolerance. The median colchicine dose was 2 mg/day (range 1–3 mg/day). Fifty-seven patients were switched to canakinumab after anakinra due to injection site reaction, systemic and local side effects, non-compliance to daily use, or lack of response. The most common side effect (n = 28, 49%) observed during anakinra was a local reaction at the injection site. Injection site reactions were observed frequently in the first 2 months of the treatment. Skin rash was observed in five of 57 patients after injection. In addition, inconvenience of daily use was the most significant obstacle during anakinra use (n = 13, 22%). Eleven out of 57 patients were switched to canakinumab due to inadequate response to anakinra. The patient’s median duration of anakinra use was 4 months (range 1–9 months).
The mean attack frequency before canakinumab was 3.4/6 months, while it was 1.2 at the last post-treatment visit (p < 0.001). Whereas the attack duration was 67.2 h before canakinumab treatment, it decreased to 14.27 h after 6 months (p < 0.001). Following canakinumab treatment, a significant decrease was observed in all acute inflammatory markers, including CRP and ESR levels (p < 0.05) (Table 2).
Four patients with amyloidosis were diagnosed with biopsy (rectal biopsy n = 4). Amyloidosis patients did not develop any side effects under treatment and tolerated the drug well. While proteinuria had a stable course in two patients, an improvement was observed in proteinuria levels in the other two patients.
The median Pras score was 3.2 (range 6–11), and after a six-month canakinumab treatment, a statistically significant improvement was observed in the Pras score (p < 0.05). Additionally, canakinumab was effective in decreasing the number of attacks, attack duration and acute-phase reactants (p < 0.001) (Table 2).
Canakinumab dose tapering and discontinuation
All patients started with 150 mg per month, and the dose and interval were not changed for the first 6 months. Although the frequency and duration of attacks decreased in 35 of 57 patients who received monthly, the drug dose interval was not extended as there was no sign of complete remission. The frequency of attacks in the group of 35 patients was 1.2 attacks/6 months. In such patients, complaints, such as joint pain and leg pain, upon exertion continued during the attack-free periods. Intermittent elevations were observed in CRP and sedimentation values during the periods between the attacks in 13 of these patients. In this patient group, there were four patients with amyloidosis.
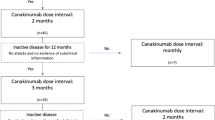
In Fig. 1, a summary of the patients is presented. After the first 6 months, the dosing interval of canakinumab was extended in 22 patients, and then treatment was discontinued in 12 of them who did not have an attack in the last six months. No subclinical acute-phase reactant elevation was observed in these patients during the attack-free period. For the remaining ten patients, dose intervals were extended to 8–12 weeks after 6 months of monthly canakinumab treatment. The median canakinumab administration interval was 2 months (2–3 months) in 22 patients whose canakinumab dose interval was extended. The median attack-free follow-up period was 6.8 months (range 3–15) in patients with extended dose intervals.
The drug was discontinued in 12 patients who were in remission as their dose interval was extended, they had no attacks, and their acute-phase tests were normal. These patients were administered monthly canakinumab for the first 6 months, then the dosing interval was increased to a median of 8 weeks (range 8–12), and no attacks were observed in the second six-month period. Three of the 12 patients whose canakinumab treatment was discontinued started monthly treatment again after their attacks recurred at 4, 5 and 7 months after the discontinuation of the therapy. The remaining nine patients are still being followed up without attacks and receive only colchicine therapy. The attack-free median follow-up time for the nine patients whose treatment with canakinumab was discontinued and under follow-up with only colchicine treatment is 9.4 months (range 4–14).
An increase in the attack frequency was observed in four of the remaining ten patients whose dose interval was extended after a median of 4 months (range 2–5) from the extension of the dose intervals. Canakinumab dose interval was rescheduled on monthly bases for four patients with two or more attacks (three patients had two attacks, and one patient had three attacks). Six patients whose extended dose intervals are still being followed up without attack. This patient group's median attack-free follow-up period was 8.6 months (range: 6–13). Table 3 compares clinical and laboratory characteristics of patients with a 4-week canakinumab dose interval versus an > 4-week dose interval. The number of attacks, amyloidosis and erysipelas-like erythema was significantly higher in the 4-week group (p < 0.05). M694V homozygous mutation was high in our FMF patients in the study group (40/57). The M694V mutation rate was 5/10 in 10 patients whose dose interval was extended and 7/12 in 12 patients who were discontinued.
Drug safety
No serious adverse events were observed during the canakinumab treatment since the beginning of the treatment, except mild infection. In this current study, no tuberculosis, opportunistic infections, a condition requiring hospitalization or parenteral antibiotics, malignancies or death occurred during the median 46-month period (range 9–64 months) of canakinumab. During the follow-up period, 12 patients developed respiratory tract infections, and throughout the pandemic period, four patients had SARS-CoV2 disease. Such patients were followed up and treated without requiring hospitalization. Weight gain was observed in three of the patients receiving canakinumab.
Discussion
In the present study, we demonstrate the real-life experiences with canakinumab dose intervals, discontinuation and long-term data in FMF patients who cannot use colchicine and anakinra for any reason. IL-1 blockers have been introduced as new alternative treatment modalities in the presence of colchicine-resistant and intolerant patients, and studies have provided their effectiveness [20,21,22,23]. The data in the literature about the dose, administration intervals, and the treatment duration of the biologicals used in FMF patients are limited. There is no consensus or guideline on this issue yet. Studies conducted on pediatric and adult patients prove the efficacy and safety of canakinumab. While these and similar studies provide information on efficacy and safety, they do not provide sufficient information on the drug's optimal duration of use and dose intervals [16, 24, 25]. In our study, dose intervals were extended in 10 of 57 patients after starting treatment and treatment was discontinued in 12 patients. While new attacks were observed in 40% of the patients whose dose interval was extended, the attacks were controlled with monthly canakinumab administration. In 25% of 12 patients whose treatment was discontinued, the drug was restarted due to an attack, and the attacks were controlled with monthly canakinumab.
The CLUSTER trial randomized adult patients to receive canakinumab 150 mg every four weeks or a placebo. Subsequently, patients with complete responses by week 16 underwent a second randomization to receive either canakinumab or placebo every eight weeks up to week 40. Remission was maintained in 46% of the patients administered the drug every eight weeks. Results from the CLUSTER trial at 72 weeks demonstrated a minimal incidence of exacerbations and reasonable control of clinical disease activity [26]. In another study, the dose intervals of pediatric FMF patients who entered remission at the end of 6 months with canakinumab treatment every 2 months were later increased to 3 months. Still, the data on the follow-up of the patients were not reported in the study [21]. A retrospective study of 14 adult FMF patients reported that 79% of their patients achieved complete clinical remission and 21% partial response within 2 months. Four patients in this study group experienced relapse, and the canakinumab interval was shortened again in two patients in remission [27].
This and similar studies instead emphasize the efficacy and safety data of canakinumab. The studies in the literature report remission in gradually increasing frequency in patients receiving IL-1 treatment. Although the remission rates in the earlier studies were higher than our findings, the efficacy and side effect profiles were consistent with our research. In studies in the literature, data on prolonging the dose intervals of drugs and discontinuing drug therapy in patients' treatment protocols in remission are limited [23]. Likewise, there are insufficient data regarding the recurrence time of the attacks and the course of the disease after restarting the treatment in patients whose drug treatment was discontinued [16, 24,25,26].
Although it is not known in detail which attack symptom (joint, serosal, etc.) responds better to which IL-1 blocker, it is stated that both drugs are effective in all attack findings and reduce the number of attacks [28, 29]. In our study, it was observed that there was a decrease in the frequency and number of attacks in almost all patients treated with canakinumab, consistent with previous efficacy studies. In addition to the frequency of attacks, a significant decrease was also shown in acute-phase reactants with IL-1 antagonists, similar to our results [8, 20, 23, 30].
Akarcan et al. administered monthly canakinumab to all patients in a pediatric FMF series of nine patients for the first month. Then they administered three doses of treatment every 2 months. After nine doses of medicine, canakinumab treatment was discontinued, and the patients were followed up for attacks. Patients who had new attacks were administered canakinumab again every 3 months. Four patients had new attacks during the follow-up and were administered continuation treatment [29]. This study had a limited number of patients and a standard treatment protocol regardless of patients' characteristics. For a disease that is very common in the real-life setting, many factors, such as comorbidities, concomitant drugs used by the patient, or the conditions the patient develops at the time of treatment (infection or similar conditions), may make it difficult to implement standard dose reduction or discontinuation protocols. There may be patient-specific differences in the approach to the treatment of FMF, which is a common disease in our country. Dose intervals may be extended for patients selected by the monitoring physician. In the present study conducted in real life, dose intervals were extended in patients who achieved complete response (remission) without being bound by any protocol at the discretion of the rheumatology physician who followed up with the patients in the outpatient clinic. The drug was discontinued in some patients whose dose intervals were 2 months or longer and remained in remission for 6 months or longer.
Factors, such as high AFR, presence of amyloidosis, family history of amyloidosis, frequency of attacks before treatment, and severity of attacks, may play a role in not extending the dosing interval, not stopping the drug, and continuing the monthly treatment. The interval was not extended in our four patients with amyloidosis, and the monthly treatment continued. Although there is more definite information about IL-1 inhibitors reducing the frequency of attacks, there are conflicting studies regarding the reduction in proteinuria in patients with amyloidosis [31,32,33]. It is unclear whether switching to anakinra or canakinumab will effectively treat problems such as progression in patients with amyloidosis during treatment and should be investigated in larger patient groups. Therefore, we think it would be appropriate to continue the use of colchicine at the maximum tolerated dose without interruption and indefinitely in patients with FMF-related amyloidosis and to add anakinra or canakinumab to colchicine treatment without extending the dose intervals. Except for amyloidosis, the other patient group in whom we could not open the dosing interval was patients with erysipelas-like erythema.
M694V homozygous mutation is associated with early-onset disease, poor prognosis, and amyloidosis [34,35,36,37]. These patient characteristics cause severe disease phenotypes and may increase the need for anti-IL-1 agents. In our study, the M694V mutation rate was high, and the drug dose intervals could be extended, or the drug could be discontinued in these patients, suggesting that this approach can be applied in patients with a milder course.
In the studies conducted with canakinumab, no side effects were observed other than mild infection, the drug was reported to be safe, and no severe side effects were observed in our study [36,37,38]. No serious adverse events were observed in the present study, and patients with SARS-CoV2 infection were treated without hospitalization.
Laboratory findings and clinical observations indicate that canakinumab may be an alternative treatment option in patients who do not respond to colchicine. Although canakinumab is effective and safe, it should be evaluated in terms of cost. The drug's duration of use, dose intervals and discontinuation conditions in FMF patients should be discussed and determined. There are limited data on the drug’s dose intervals and discontinuation in the adult patient group. If no attacks and subclinical inflammation are observed after starting canakinumab, dose intervals can be doubled initially and then tripled if no new attack occurs within one year [38]. As the present study is a real-life study, which included a limited number of cases and is conducted in routine outpatient clinic conditions, it may contribute to the literature in this respect. We could extend the dose intervals or discontinue the drug in 22 of 57 patients included in the study. The attacks that recurred in some patients after discontinuing the medication or extending the dosing interval were retaken under control by switching back to the monthly administration or the previous dose interval.
The limitations of the present study include its retrospective nature, lack of a protocol for extending canakinumab dose interval and discontinuation of the drug, and the limited number of cases. Different physicians' diverse practices in this real-life observational study can be another limitation. Additionally, FMF differs in behavior from person to person and is a patient-specific approach during treatment.
Conclusion
Canakinumab dose intervals can be extended, and follow-up without medication may be possible for eligible FMF patients. However, data from prospective studies with more cases and a consensus on drug dose optimisation are still needed in patients with FMF. How to extend dose intervals and which patients' treatment will be discontinued may be defined, and protocols can be created for a standardized approach.
Data availability
The authors of current study confirms that the findings of this study are available and resented with the manuscript. All raw data and findings available with corresponding author and can be provided upon request.
References
Grateau G, Duruoz MT (2010) Autoinflammatory conditions: when to suspect? How to treat? Best Pract Res Clin Rheumatol 24(3):401–411. https://doi.org/10.1016/j.berh.2009.12.009
Karabulut Y, Gezer HH, Duruoz MT (2022) Canakinumab is effective in patients with familial mediterranean fever resistant and intolerant to the colchicine and/or anakinra treatment. Rheumatol Int 42(1):81–86. https://doi.org/10.1007/s00296-021-04997-y
Ozen S (2018) Update on the epidemiology and disease outcome of familial mediterranean fever. Best Pract Res Clin Rheumatol 32(2):254–260. https://doi.org/10.1016/j.berh.2018.09.003
Ozen S, Bilginer Y (2014) A clinical guide to autoinflammatory diseases: familial mediterranean fever and next-of-kin. Nat Rev Rheumatol 10(3):135–147. https://doi.org/10.1038/nrrheum.2013.174
Ozen S, Sag E, Ben-Chetrit E, Gattorno M, Gul A, Hashkes PJ, Kone-Paut I, Lachmann HJ, Tsitsami E, Twilt M, Benedetti F, Kuemmerle-Deschner JB (2021) Defining colchicine resistance/intolerance in patients with familial Mediterranean fever: a modified-Delphi consensus approach. Rheumatology 60(8):3799–3808. https://doi.org/10.1093/rheumatology/keaa863
Tufan A, Lachmann HJ (2020) Familial Mediterranean fever, from pathogenesis to treatment: a contemporary review. Turk J Med Sci 50(SI-2):1591–1610. https://doi.org/10.3906/sag-2008-11
Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, Kastner DL (2006) The B30.2 domain of pyrin, the familial mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci U S A 103(26):9982–9987. https://doi.org/10.1073/pnas.0602081103
Yasar Bilge S, Sari I, Solmaz D, Senel S, Emmungil H, Kilic L, Yilmaz Oner S, Yildiz F, Yilmaz S, Ersozlu Bozkirli D, Aydin Tufan M, Yilmaz S, Yazisiz V, Pehlivan Y, Bes C, Yildirim Cetin G, Erten S, Gonullu E, Sahin F, Akar S, Aksu K, Kalyoncu U, Direskeneli H, Erken E, Kisacik B, Sayarlioglu M, Cinar M, Kasifoglu T (2019) The distribution of MEFV mutations in Turkish FMF patients: multicenter study representing results of Anatolia. Turk J Med Sci 49(2):472–477. https://doi.org/10.3906/sag-1809-100
Seshadri S, Duncan MD, Hart JM, Gavrilin MA, Wewers MD (2007) Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1beta processing and release. J Immunol 179(2):1274–1281. https://doi.org/10.4049/jimmunol.179.2.1274
Repa A, Bertsias GK, Petraki E, Choulaki C, Vassou D, Kambas K, Boumpas DT, Goulielmos G, Sidiropoulos P (2015) Dysregulated production of interleukin-1beta upon activation of the NLRP3 inflammasome in patients with familial Mediterranean fever. Hum Immunol 76(7):488–495. https://doi.org/10.1016/j.humimm.2015.06.007
Leung YY, Yao Hui LL, Kraus VB (2015) Colchicine-update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 45(3):341–350. https://doi.org/10.1016/j.semarthrit.2015.06.013
Satis H, Armagan B, Bodakci E, Atas N, Sari A, Yasar Bilge NS, Yapar D, Bilici Salman R, Yardimci GK, Babaoglu H, Kilic L, Goker B, Haznedaroglu S, Kasifoglu T, Kalyoncu U, Tufan A (2020) Colchicine intolerance in FMF patients and primary obstacles for optimal dosing. Turk J Med Sci 50(5):1337–1343. https://doi.org/10.3906/sag-2001-261
Sonmez HE, Batu ED, Ozen S (2016) Familial mediterranean fever: current perspectives. J Inflamm Res 9:13–20. https://doi.org/10.2147/JIR.S91352
Ozen S, Bilginer Y, Aktay Ayaz N, Calguneri M (2011) Anti-interleukin 1 treatment for patients with familial mediterranean fever resistant to colchicine. J Rheumatol 38(3):516–518. https://doi.org/10.3899/jrheum.100718
Ozen S, Demirkaya E, Erer B, Livneh A, Ben-Chetrit E, Giancane G, Ozdogan H, Abu I, Gattorno M, Hawkins PN, Yuce S, Kallinich T, Bilginer Y, Kastner D, Carmona L (2016) EULAR recommendations for the management of familial mediterranean fever. Ann Rheum Dis 75(4):644–651. https://doi.org/10.1136/annrheumdis-2015-208690
Cetin P, Sari I, Sozeri B, Cam O, Birlik M, Akkoc N, Onen F, Akar S (2015) Efficacy of interleukin-1 targeting treatments in patients with familial mediterranean fever. Inflammation 38(1):27–31. https://doi.org/10.1007/s10753-014-0004-1
Pras E, Livneh A, Balow JE Jr, Pras E, Kastner DL, Pras M, Langevitz P (1998) Clinical differences between North African and Iraqi Jews with familial mediterranean fever. Am J Med Genet 75(2):216–219
Erden A, Batu ED, Sari A, Sonmez HE, Armagan B, Demir S, Firat E, Bilginer Y, Bilgen SA, Karadag O, Kalyoncu U, Kiraz S, Ertenli I, Ozen S, Akdogan A (2018) Which definition should be used to determine colchicine resistance among patients with familial Mediterranean fever? Clin Exp Rheumatol 36(6 Suppl 115):97–102
Kisla Ekinci RM, Balci S, Dogruel D, Altintas DU, Yilmaz M (2019) Canakinumab in children with familial mediterranean fever: a single-center retrospective analysis. Paediatr Drugs 21(5):389–395. https://doi.org/10.1007/s40272-019-00354-6
Kuijk LM, Govers AM, Frenkel J, Hofhuis WJ (2007) Effective treatment of a colchicine-resistant familial mediterranean fever patient with anakinra. Ann Rheum Dis 66(11):1545–1546. https://doi.org/10.1136/ard.2007.071498
Sag E, Akal F, Atalay E, Akca UK, Demir S, Demirel D, Batu ED, Bilginer Y, Ozen S (2020) Anti-IL1 treatment in colchicine-resistant paediatric FMF patients: real life data from the HELIOS registry. Rheumatology 59(11):3324–3329. https://doi.org/10.1093/rheumatology/keaa121
Mitroulis I, Skendros P, Oikonomou A, Tzioufas AG, Ritis K (2011) The efficacy of canakinumab in the treatment of a patient with familial Mediterranean fever and longstanding destructive arthritis. Ann Rheum Dis 70(7):1347–1348. https://doi.org/10.1136/ard.2010.146878
Eroglu FK, Besbas N, Topaloglu R, Ozen S (2015) Treatment of colchicine-resistant familial mediterranean fever in children and adolescents. Rheumatol Int 35(10):1733–1737. https://doi.org/10.1007/s00296-015-3293-2
Gul A, Ozdogan H, Erer B, Ugurlu S, Kasapcopur O, Davis N, Sevgi S (2015) Efficacy and safety of canakinumab in adolescents and adults with colchicine-resistant familial mediterranean fever. Arthritis Res Ther 17:243. https://doi.org/10.1186/s13075-015-0765-4
Brik R, Butbul-Aviel Y, Lubin S, Ben Dayan E, Rachmilewitz-Minei T, Tseng L, Hashkes PJ (2014) Canakinumab for the treatment of children with colchicine-resistant familial mediterranean fever: a 6-month open-label, single-arm pilot study. Arthritis Rheumatol 66(11):3241–3243. https://doi.org/10.1002/art.38777
Ozen S, Ben-Cherit E, Foeldvari I, Amarilyo G, Ozdogan H, Vanderschueren S, Marzan K, Kahlenberg JM, Dekker E, De Benedetti F, Kone-Paut I (2020) Long-term efficacy and safety of canakinumab in patients with colchicine-resistant familial mediterranean fever: results from the randomised phase III CLUSTER trial. Ann Rheum Dis 79(10):1362–1369. https://doi.org/10.1136/annrheumdis-2020-217419
Laskari K, Boura P, Dalekos GN, Garyfallos A, Karokis D, Pikazis D, Settas L, Skarantavos G, Tsitsami E, Sfikakis PP (2017) Longterm beneficial effect of canakinumab in colchicine-resistant familial mediterranean fever. J Rheumatol 44(1):102–109. https://doi.org/10.3899/jrheum.160518
Hentgen V, Vinit C, Fayand A, Georgin-Lavialle S (2020) The use of interleukine-1 inhibitors in familial mediterranean fever patients: a narrative review. Front Immunol 11:971. https://doi.org/10.3389/fimmu.2020.00971
Eren Akarcan S, Dogantan S, Edeer Karaca N, Aksu G, Kutukculer N (2020) Successful management of colchicine resistant familial mediterranean fever patients with a standardized canakinumab treatment protocol: a case series and literature review. Rheumatol Int 40(1):161–168. https://doi.org/10.1007/s00296-019-04366-w
Babaoglu H, Varan O, Kucuk H, Atas N, Satis H, Salman R, Ozturk MA, Goker B, Tufan A, Haznedaroglu S (2020) Effectiveness of canakinumab in colchicine- and anakinra-resistant or -intolerant adult familial mediterranean fever patients: a single-center real-life study. J Clin Rheumatol 26(1):7–13. https://doi.org/10.1097/RHU.0000000000000873
Sahin A, Derin ME, Albayrak F, Karakas B, Karagoz Y (2020) Assessment of effectiveness of anakinra and canakinumab in patients with colchicine-resistant/unresponsive familial Mediterranean fever. Adv Rheumatol 60(1):12. https://doi.org/10.1186/s42358-020-0117-1
Akar S, Cetin P, Kalyoncu U, Karadag O, Sari I, Cinar M, Yilmaz S, Onat AM, Kisacik B, Erden A, Balkarli A, Kucuksahin O, Oner SY, Senel S, Tufan A, Direskeneli H, Oksuz F, Pehlivan Y, Bayindir O, Keser G, Aksu K, Omma A, Kasifoglu T, Unal AU, Yildiz F, Balci MA, Yavuz S, Erten S, Ozgen M, Sayarlioglu M, Dogru A, Yildirim G, Oner FA, Tezcan ME, Pamuk ON, Onen F (2018) Nationwide experience with off-label use of interleukin-1 targeting treatment in familial mediterranean fever patients. Arthritis Care Res 70(7):1090–1094. https://doi.org/10.1002/acr.23446
Lane T, Wechalekar AD, Gillmore JD, Hawkins PN, Lachmann HJ (2017) Safety and efficacy of empirical interleukin-1 inhibition using anakinra in AA amyloidosis of uncertain aetiology. Amyloid 24(3):189–193. https://doi.org/10.1080/13506129.2017.1352503
Shinar Y, Livneh A, Langevitz P, Zaks N, Aksentijevich I, Koziol DE, Kastner DL, Pras M, Pras E (2000) Genotype-phenotype assessment of common genotypes among patients with familial mediterranean fever. J Rheumatol 27(7):1703–1707
Mukhin NA, Kozlovskaya LV, Bogdanova MV, Rameev VV, Moiseev SV, Simonyan A (2015) Predictors of AA amyloidosis in familial mediterranean fever. Rheumatol Int 35(7):1257–1261. https://doi.org/10.1007/s00296-014-3205-x
Ben-Zvi I, Kukuy O, Giat E, Pras E, Feld O, Kivity S, Perski O, Bornstein G, Grossman C, Harari G, Lidar M, Livneh A (2017) Anakinra for colchicine-resistant familial mediterranean fever: a randomized, double-blind placebo-controlled trial. Arthritis Rheumatol 69(4):854–862. https://doi.org/10.1002/art.39995
Kosan C, Diri N, Cayir A, Turan MI (2015) Clinical profile of familial mediterranean fever in a paediatric population in Eastern Turkey. West Indian Med J 65(2):281–286. https://doi.org/10.7727/wimj.2014.173
Kavrul Kayaalp G, Sozeri B, Sonmez HE, Demir F, Cakan M, Ozturk K, Karadag SG, Otar Yener G, Ozdel S, Baglan E, Celikel E, Sahin N, Gezgin Yildirim D, Eker Omeroglu R, Aktay Ayaz N, Pe RARG (2022) Adherence to best practice consensus guidelines for familial Mediterranean fever: a modified Delphi study among paediatric rheumatologists in Turkey. Rheumatol Int 42(1):87–94. https://doi.org/10.1007/s00296-020-04776-1
Funding
This study was not funded by any organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author have no affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Ethical approval
Current study approved by the Committee on the Human Research Ethics.
Informed consent
Current study conducted retrospectively and Informed consent was not obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karabulut, Y., Gezer, H.H., Öz, N. et al. Real-life data on tapering or discontinuation of canakinumab therapy in patients with familial Mediterranean fever. Rheumatol Int 42, 2211–2219 (2022). https://doi.org/10.1007/s00296-022-05199-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05199-w