Abstract
Investigate the natural history of urinary incontinence (UI) in systemic sclerosis (SSc) and assess its impact on quality of life (QoL). A longitudinal, international observational study followed 189 patients with SSc for a median duration of 5 years (IQR: 4.8–5.3). Presence, subtype and severity of UI, hospital admission and QoL were assessed using serial self-administered questionnaires. Mortality data came from national death registries. Multilevel mixed-effect logistic regressions explored factors associated with UI. Cox models adjusted the effects of UI on hospitalization and death for age, sex and subtype of SSc. Mean annual rates of new-onset UI and remission were 16.3% (95%CI 8.3%–24.2%) and 20.8% (95%CI 12.6–29.1), respectively. Among UI patients, 57.9% (95%CI 51.8–64.0) changed from one UI subtype to another. Between annual questionnaires, the severity of UI was the same in 51.1% (95%CI 40.8–61.4), milder or resolved in 35.2% (95%CI 25.3–44.9), and worse in 13.8% (95%CI 6.7–20.9). Anti-centromere antibodies, digestive symptoms, sex, age, neurological or urological comorbidities, diuretics and puffy fingers were all associated with UI. The two strongest predictors of UI and UI subtypes were a recent UI episode and the subtype of previous leakage episodes. UI at inclusion was not associated with hospital admission (adjusted HR: 1.86; 95%CI 0.88–3.93), time to death (aHR: 0.84; 95%CI 0.41–1.73) or change in QoL over time. Self-reported UI among SSc patients is highly dynamic: it waxes and wanes, changing from one subtype to another over time.
Similar content being viewed by others
Introduction
Urinary incontinence (UI) affects the quality of life (QoL) [1] and work productivity [2], increases the risks of falls [3], mood disorders [4, 5] and institutionalisation [6], and it has been associated with higher mortality [7, 8]. Thus, UI has a significant impact on individuals and their medical care [9].
UI is now recognised as a highly prevalent condition in cases of systemic sclerosis (SSc), affecting 52–63% of patients [10, 11]. Epidemiology and associated factors are very different to those of UI in the general population or in other conditions [10]. Thus, its pathogenesis possibly involves SSc-specific mechanisms that have yet to be fully elucidated [12]. Fibrosis and vascular anomalies have been reported in the bladder of SSc patients [13,14,15,16,17,18]. However, an autopsy study found no more fibrosis in the bladders of sclerodermic patients, than in controls [19]. Alteration of the nervous system, notably dysfunction of the parasympathetic system seen in SSc, could play a role in UI [16, 18]. But Minervini et al. failed to link autonomic function and urinary symptoms [16]. Menopause can appear earlier in SSc and might play a role in women [20]. Finally, an antibody-mediated mechanism is supported by in vitro analysis [21], and parallels made with other rheumatologic diseases [22].
To date, there were no data available on the natural history of UI in SSc. Some reports have found a higher prevalence of UI in the limited form of SSc or among patients having anti-centromere antibodies (ACA) [11], whereas others have found an association with anti-topoisomerase I (anti-Scl70) antibodies [10], with a very different prognosis [23]. Thus, we aimed to investigate the natural history of UI in SSc, the factors influencing it and explore UI as an indicator of unfavourable outcomes by focusing on QoL, physical well-being and the risks of hospital admission or death.
Patients and methods
Study overview
This international, prospective, observational study enrolled patients suffering from SSc at four European tertiary hospital centres from January 2013 to December 2015. Patients underwent three annual evaluations of their lower urinary tract symptoms (LUTS) by questionnaires in two centers, two annual evaluations in one center and one evaluation in the last center. All patients were then followed until death or to 31 December 2019. The study complies with the Declaration of Helsinki. Locally appointed ethics committee has approved the research protocol. Written informed consent has been obtained from all patients included in the study. The following sections summarise our cohort population data, inclusion process and variable management, which have been published previously [11, 24].
Study outcomes
The main outcome was continence evolution at annual clinical evaluations. The secondary outcomes were first UI episode, QoL, first hospital admission, number of admission, length of stay, and death.
Scenarios of urinary continence evolution between study visits were “new-onset UI”, “resolved UI symptoms”, “worsening of UI symptoms”, “decreasing UI symptoms”, and “changing UI subtype”. The first described patients continent of urine in the previous study visit, who complained of UI in the follow-up visit. The second described patients continent of urine, who used to complain of UI in the last study visit. The three last scenarios refer to patients with UI in two study visits, who changed the frequency of UI episodes (from daily to monthly for example), or changed the subtype of UI (changing from stress to urge UI for example).
Study population
Eligible patients were aged 18 or older and satisfied the ACR/EULAR 2013 SSc criteria [25]. They were included consecutively at each participating centre. Those unable or unwilling to follow the study’s rules, end-of-life patients, pregnant women and anuric patients were excluded.
Symptoms and measurement
At inclusion and each annual study visit, participants completed a thirty-minute self-administered questionnaire on their LUTS, QoL, functional status, disease activity, medication, medical history and demographic characteristics. Patients were classified as having the limited or diffuse cutaneous form of SSc, according to Le Roy et al. [26].
At each study visit, UI was characterised using the International Consultation on Incontinence Modular Questionnaires (ICIQ-FLUTS and ICIQ-MLUTS) [27]. These explore the last four weeks’ LUTS and use standards recommended by the International Continence Society [28]. Participants could also complete a between-visits form for new episodes of UI occurring between study visits. UI was defined as any involuntary leakage of urine and was subdivided into stress (SUI), urge (UUI) and mixed UI. Leakage severity was further stratified according to leakage frequency (monthly, weekly or daily leakage).
The 36-item short-form health survey (SF-36) [29, 30] was summarised into its physical component (PCS) and mental component (MCS), and the Incontinence Quality of Life (I-QOL) questionnaire was provided as a single, transformed scale ranging from 0 to 100 points [31]. Functional activities were explored using the Scleroderma modified Health Assessment Questionnaire and Disability Index (SSc-HAQ-DI) and the Cochin scale [32].
First hospital admission, length of stay and number of admissions between study visits were collected using self-administered questionnaires and participating hospitals’ electronic databases. Data on the date of death were obtained from national death registries and each participating hospital’s electronic databases until the end of December 2019. Each participating centre stratified the cause of death into SSc-related and SSc-unrelated based on their expertise.
Statistics
The initial sample involved five institutions and was calculated to demonstrate differences in UI prevalence between SSc subtypes at the baseline evaluation [11]. However, only four of the five institutions took part in the prospective cohort, which was therefore composed of 189 SSc individuals.
Comparisons between the continent and incontinent participants were performed using the appropriate chi-squared test or Fischer’s exact test for categorical variables. The two-sided Mann–Whitney test was used for continuous variables. Variables for the same individual measured on two occasions (QoL, HAQ-DI and Cochin scale) were compared using the paired Wilcoxon signed-rank test.
To evaluate factors associated with the natural history of UI, we calculated multilevel mixed-effect logistic regressions accounting for repeated measures and patients being clustered in different study centres. Analyses were repeated for any evolutions in UI scenarios at annual clinical evaluations. A random intercept was given to any individual to account for repeated measures. Other models explored risks by UI subtype and severity (dependent variables). Potential associated factors were chosen based on our previous publications [11, 24]. Considering the small sample size and the number of events, we performed exploratory univariate analyses and decided not to perform multivariate analyses.
The unadjusted effects of UI on time-dependent outcomes (first UI episode, first hospital admission or death) were analysed using Kaplan–Meier survival analysis and unweighted, two-sided, log-rank tests to compare groups. Participants with unknown status on 31 December 2019 were considered lost to follow-up and were censored from their last medical visit. Multivariate Cox models were used to adjust for age, sex and type of SSc. The same analyses were performed according to the subtype and severity of UI and SSc-related death.
The significance level was set at 5%, and all analyses were performed using STATA statistical software, version 12.0 (StataCorp LP, College Station, TX, USA).
Results
A total of 189 SSc patients were followed for a median duration of 5 years (IQR: 4.8–5.3) (Fig. 1). Their second and third study visits were at a median of 1.1 (IQR: 1.0–1.1) and 2.1 (IQR: 2.0–2.7) years after inclusion, respectively. Patients’ principal characteristics are given in Table 1.
Urinary incontinence status and severity over time
Of 189 patients, 118 were followed for their urinary symptoms for one year and 86 for two years (Fig. 1). The time to first UI episode is presented in Fig. 2. After a steep drop due to patients with UI at inclusion, the curve followed a shallower decrease due to new UI episodes among patients continent of urine at study inclusion.
Time to first urinary incontinence episode shown against urinary continence status at admission and for the complete study-cohort. After a steep drop due to patients with UI at inclusion (dash), the curve shows a shallower decrease due to new UI episodes among patients continent of urine at study inclusion (long dash-dot). UI: urinary incontinence
Continence status changed for 34 (29%) of 118 patients between their inclusion and their second visit; it changed for 22 (26%) of 86 patients between their second and third study visits. The yearly rate of new-onset UI was 16.3% (95% CI 8.3%–24.2%). Each year, the symptoms of 20.8% (95% CI 12.6–29.1) of patients suffering from UI resolved, 57.9% (95% CI 51.8–64.0) changed from one subtype of UI to another (e.g., from stress to urge), and 34.2% (95% CI 28.4–40.1) kept the same UI subtype (Fig. 3). Between annual evaluations, the severity of UI was the same in 51.1% (95% CI 40.8–61.4) of patients, milder or resolved in 35.2% (95% CI 25.3–44.9) and worse in 13.8% (95% CI 6.7–20.9) (Fig. 4).
Prevalence and types of urinary incontinence (UI) between first and second study visits (panel A) and between second and third study visits (panel B). Small boxes and arrows show the percentages of patients whose subtype of UI had changed. * Due to missing data, the sums of the proportions do not equal 100 per cent. UI: urinary incontinence
Prevalence and severity of urinary incontinence (UI) between first and second study visits (panel A) and between second and third study visits (panel B). Small boxes and arrows show the percentages of patients whose severity of UI had changed. * Due to missing data, the sums of the proportions do not equal 100 per cent. UI: urinary incontinence
Factors associated with the evolution of UI in SSc
Factors associated with the natural history of UI are reported in Supplementary Table S1 and S2. Presenting with puffy fingers on clinical evaluation increased the composite risk of “new-onset UI” or “worsening of UI symptoms” (OR 2.6; 95% CI 1.2–5.9). None of the factors was constantly associated with the risk of either “new-onset UI” or “worsening of UI symptoms”.
The combined chance of being attributed an outcome of “decreasing severity of UI”, “resolving UI symptoms” or “maintaining continence” decreased with previous episodes of SUI (OR 0.2; 95% CI 0.1–0.4) or UUI (OR 0.2; 95% CI 0.1–0.4), the presence of ACA (OR 0.1; 95% CI 0.04–0.5) or digestive symptoms (OR 0.2; 95% CI 0.1–0.9), and it was higher in men (OR 9.2; 95% CI 1.2–73.4). These factors’ effects on the risk of “decreasing severity of UI” or the risk of “resolving UI symptoms” were inconstant (Supplementary Table S1).
The risk of UUI at subsequent visits increased with previous episodes of UUI (OR 18.6; 95% CI 8.5–40.6) or SUI (OR 3.7; 95% CI 1.1–12.9), ACA positivity (OR 16.9; 95% CI 2.5–116), digestive symptoms (OR 32.4; 95% CI 3.3–320), an age above than median (OR 9.5; 95% CI 1.5–59.6) and higher frequencies of episodes of UI (Supplementary Table S2). The risk of SUI at the subsequent visit increased with previous episodes of SUI (OR 19.1; 95% CI 8.8–41.6) or UUI (OR 8.4; 95% CI 2.3–31.3) and with higher frequencies of episodes of UI. However, the risk was lower for men (OR 0.01; 95% CI 0.001–0.35).
Age, neurological palsy, urological comorbidities, UUI, SUI, diuretic treatments, ACA positivity, the limited cutaneous form of SSc and the previous severity of episodes of UI were inconstantly associated with monthly, weekly or daily episodes of UI (Supplementary Table S2).
Quality of life and disability
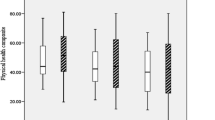
Except for the “physical functioning” domain of SF-36, patients’ QoL remained stable from the first to the third study visit, and there were no statistical differences between patients with and without UI. This was also true for changes in disability between these two evaluations and between the continent and incontinent patients (Supplementary Table S3).
Hospital admission and mortality
Between their inclusion and their third study visit, 43 (36%) patients were hospitalised at least once, with a median length of stay of 8 days (IQR: 5–18). UI at inclusion was not associated with time to first hospital admission in our univariate (HR 1.17; 95% CI 0.63–2.16) or adjusted analyses (HR 1.27; 95% CI 0.67–2.42). This was also true when considering different subtypes of UI and severity (Table 2).
Thirty deaths (19%) occurred during the median follow-up time of 5 years (IQR: 4.8–5.3), of which 22 (73%) were deemed to be SSc-related. UI at baseline was not associated with time to death in either our univariate (HR 0.97; 95% CI 0.49–1.92) or adjusted analyses (HR 0.94; 95% CI 0.46–1.91). There were also no associations between UI severity or UI subtype and death from any cause (Table 2) or SSc-related death (data not shown).
Discussion
This longitudinal, prospective, international multicentre study demonstrated that self-reported urinary incontinence (UI) among systemic sclerosis (SSc) patients is a highly dynamic phenomenon. Over a period of two years, almost 40% of participants changed their urinary status, and an episode of urine leakage (transient or persistent) affected the majority of patients. Nevertheless, compared to continent patients, UI was not associated with an unfavourable change in QoL or functional status over time or with an increased risk of hospital admission or death in SSc.
In contrast to previous beliefs that UI is a binary and constant disease, there is growing evidence that UI changes over time, with active and inactive phases. In the general population, the annual incidence of new-onset UI is 2%–10% among men and 3%–20% among women, with remission rates of 27%–32% and 3%–12%, respectively [33,34,35]. Thus, patients may experience periods of incontinence followed by periods of continence [36]. After 50–60 years old, rates of new-onset UI are lower than rates of remission, leading to a higher overall prevalence of UI in older individuals [37]. Although the present study had no control group, incidence rates of UI in SSc patients seemed to be higher than in the general population, notably for younger participants. This higher level of new-onset UI came with the previously observed higher prevalence of UI in SSc patients [10, 11].
Although UI is commonly divided into clinically independent subtypes, about a quarter of SSc patients in the present study changed from one UI subtype to another between annual visits. This has also been observed in longitudinal studies of women without SSc [35, 38]. Furthermore, the treatment of one component of mixed UI (e.g. SUI by medication or surgery) may result in the remission of the other component (UUI) [35]. Thus, the boundary between UI subtypes appears to be narrow.
Due to its dynamic nature, UI must be reassessed often (at least once a year) among SSc patients. This reassessment is important to adapt UI management on its severity and subtype and to look for factors that might influence or predict its evolution. Although results were inconstant across the different natural history scenarios of UI over time, several factors—including ACA antibodies, digestive symptoms and, to a lesser extent, sex, age, neurological palsy, urological comorbidities, diuretic treatments, the limited cutaneous form of SSc and the presence of puffy fingers on clinical evaluation—appeared to be potentially associated with UI. Nevertheless, the two strongest predictors of UI and its subtypes were a recent UI episode and the subtype of the previous leaking episodes (SUI or UUI). Understanding the factors that influence the evolution of UI, and which phases of UI have the most significant health effects, will help to reassure some patients, provide tailored treatments for severe episodes of UI that are unlikely to resolve, and also give prevention based on risk factors.
The present study had some limitations. First, as previously mentioned, because no controls were included, it was impossible to confirm whether SSc patients had higher new-onset or remission rates than the general population. Second, UI diagnoses were based on self-reported questionnaires without objective measures. The ICIQ questionnaires have nevertheless been correlated with objective measures of UI in other studies [39, 40]. Third, except for the corticosteroids, no information on suppressive medication was available in the study. Finally, the sample size was small, and the analysis of each scenario of continence evolution over time was restricted to subgroups (e.g. patients with UI for symptoms resolution). This affected the study’s power to detect influencing factors.
In conclusion, UI is a dynamic condition in SSc that waxes and wanes, eventually changing from one form to another subtype.
Data availability
The database is freely available on request at: gregor.john@rhne.ch.
Abbreviations
- ACA:
-
Anti-centromere antibodies
- HAQ-DI:
-
Health Assessment Questionnaire Disability Index
- ICIQ-FLUTS:
-
International Conference on Incontinence Questionnaire—Female Lower Urinary Tract Symptoms
- ICIQ-MLUTS:
-
International Conference on Incontinence Questionnaire—Male Lower Urinary Tract Symptoms
- I-QOL:
-
Incontinence Quality of Life (questionnaire)
- lcSSc:
-
Limited cutaneous form of Systemic Sclerosis
- LUTS:
-
Lower Urinary Tract Symptoms
- Scl70:
-
Antibody directed against topoisomerase I
- SF-36:
-
Short Form 36 (questionnaire)
- UI:
-
Urinary incontinence
References
Abrams P, Kelleher CJ, Kerr LA, Rogers RG (2000) Overactive bladder significantly affects quality of life. Am J Manag Care 6:S580–S590
Sexton CC, Coyne KS, Vats V, Kopp ZS, Irwin DE, Wagner TH (2009) Impact of overactive bladder on work productivity in the United States: results from EpiLUTS. Am J Manag Care 15:S98–S107
Chiarelli PE, Mackenzie LA, Osmotherly PG (2009) Urinary incontinence is associated with an increase in falls: a systematic review. Aust J Physiother 55:89–95. https://doi.org/10.1016/s0004-9514(09)70038-8
Dugan E, Cohen SJ, Bland DR, Preisser JS, Davis CC, Suggs PK, McGann P (2000) The association of depressive symptoms and urinary incontinence among older adults. J Am Geriatr Soc 48:413–416. https://doi.org/10.1111/j.1532-5415.2000.tb04699.x
Zorn BH, Montgomery H, Pieper K, Gray M, Steers WD (1999) Urinary incontinence and depression. J Urol 162:82–84. https://doi.org/10.1097/00005392-199907000-00020
Luppa M, Luck T, Weyerer S, Konig HH, Brahler E, Riedel-Heller SG (2010) Prediction of institutionalization in the elderly. A systematic review. Age Ageing 39:31–38. https://doi.org/10.1093/ageing/afp202
John G, Bardini C, Combescure C, Dallenbach P (2016) Urinary incontinence as a predictor of death: a systematic review and meta-analysis. PLoS ONE 11:e0158992. https://doi.org/10.1371/journal.pone.0158992
John G, Bardini C, Megevand P, Combescure C, Dallenbach P (2016) Urinary incontinence as a predictor of death after new-onset stroke: a meta-analysis. Eur J Neurol 23:1548–1555. https://doi.org/10.1111/ene.13077
Yang E, Lisha NE, Walter L, Obedin-Maliver J, Huang AJ (2018) Urinary incontinence in a national cohort of older women: implications for caregiving and care dependence. J Womens Health (Larchmt) 27:1097–1103. https://doi.org/10.1089/jwh.2017.6891
Pacini G, Paolino S, Trombetta AC, Goegan F, Pizzorni C, Alessandri E, Patanè M, Gotelli E, Ferrari G, Cattelan F, Ghio M, Casabella A, Smith V, Cutolo M (2020) Lower urinary tract symptoms in systemic sclerosis: a detailed investigation. Rheumatology (Oxford) 59:1315–1324. https://doi.org/10.1093/rheumatology/kez438
John G, Allanore Y, Polito P, Piantoni S, Fredi M, Avouac J, Franceschini F, Truchetet ME, Cozzi F, Airo P, Chizzolini C (2017) The limited cutaneous form of systemic sclerosis is associated with urinary incontinence: an international multicentre study. Rheumatology (Oxford) 56:1874–1883. https://doi.org/10.1093/rheumatology/kex230
John G (2020) Systemic sclerosis and urinary symptoms: a complex pathophysiology. Clin Rheumatol 39:5–8. https://doi.org/10.1093/rheumatology/kex230
Beigelman PM, Goldner F Jr, Bayles TB (1953) Progressive systemic sclerosis (scleroderma). N Engl J Med 249:45–58. https://doi.org/10.1056/NEJM195307092490201
Leinwand I, Duryee AW, Richter MN (1954) Scleroderma; based on a study of over 150 cases. Ann Intern Med 41:1003–1041. https://doi.org/10.7326/0003-4819-41-5-1003
La Civita L, Fiorentini L, Tognetti A, Pasero G, Ferri C (1998) Severe urinary bladder involvement in systemic sclerosis. Case report and review of the literature. Clin Exp Rheumatol 16:591–593
Minervini R, Morelli G, Minervini A, Pampaloni S, Tognetti A, Fiorentini L, Ciompi ML (1998) Bladder involvement in systemic sclerosis: urodynamic and histological evaluation in 23 patients. Eur Urol 34:47–52. https://doi.org/10.1159/000019678
Gong R, Xia Z (2019) Collagen changes in pelvic support tissues in women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol 234:185–189. https://doi.org/10.1016/j.ejogrb.2019.01.012
Bertinotti L, Bracci S, Nacci F, Colangelo N, Del Rosso A, Casale R, Pignone A, Matucci-Cerinic M (2004) The autonomic nervous system in systemic sclerosis. A review Clin Rheumatol 23:1–5. https://doi.org/10.1007/s10067-003-0812-4
D’Angelo WA, Fries JF, Masi AT, Shulman LE (1969) Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 46:428–440. https://doi.org/10.1016/0002-9343(69)90044-8
Zigman Jessica YJ, John T, Tajnoos Y (2017) Scleroderma and pelvic organ prolapse: a multidisciplinary approach to patient care and surgical planning. J Gynecol Surg 33:198. https://doi.org/10.1089/gyn.2017.0014
Singh J, Mehendiratta V, Del Galdo F, Jimenez SA, Cohen S, DiMarino AJ, Rattan S (2009) Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 297:G1206–G1213. https://doi.org/10.1152/ajpgi.00286.2009
Wang F, Jackson MW, Maughan V, Cavill D, Smith AJ, Waterman SA, Gordon TP (2004) Passive transfer of Sjogren’s syndrome IgG produces the pathophysiology of overactive bladder. Arthritis Rheum 50:3637–3645. https://doi.org/10.1002/art.20625
Brand M, Hollaender R, Rosenberg D, Scott M, Hunsche E, Tyndall A et al (2015) An observational cohort study of patients with newly diagnosed digital ulcer disease secondary to systemic sclerosis registered in the EUSTAR database. Clin Exp Rheumatol 33:S47-54
John G, Avouac J, Piantoni S, Polito P, Fredi M, Cozzi F, Airò P, Truchetet ME, Franceschini F, Allanore Y, Chizzolini C (2018) Prevalence and disease-specific risk factors for lower urinary tract symptoms in systemic sclerosis: an international multicenter study. Arthritis Care Res (Hoboken) 70:1218–1227. https://doi.org/10.1002/acr.23454
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A et al (2013) 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 65:2737–2747. https://doi.org/10.1002/art.38098
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, Rowell N, Wollheim F (1988) Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15:202–205
Abrams P, Avery K, Gardener N, Donovan J (2006) The international consultation on incontinence modular questionnaire: http://www.iciq.net. J Urol 175:1063–1066. https://doi.org/10.1016/S0022-5347(05)00348-4
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49. https://doi.org/10.1016/s0090-4295(02)02243-4
Cossutta R, Zeni S, Soldi A, Colombelli P, Belotti Masserini A, Fantini F (2002) Evaluation of quality of life in patients with systemic sclerosis by administering the SF-36 questionnaire. Reumatismo 54:122–127. https://doi.org/10.4081/reumatismo.2002.122
Perneger TV, Leplege A, Etter JF, Rougemont A (1995) Validation of a French-language version of the MOS 36-Item Short Form Health Survey (SF-36) in young healthy adults. J Clin Epidemiol 48:1051–1060. https://doi.org/10.1016/0895-4356(94)00227-h
Patrick DL, Martin ML, Bushnell DM, Marquis P, Andrejasich CM, Buesching DP (1999) Cultural adaptation of a quality-of-life measure for urinary incontinence. Eur Urol 36:427–435. https://doi.org/10.1159/000020026
Rannou F, Poiraudeau S, Berezne A, Baubet T, Le-Guern V, Cabane J, Guillevin L, Revel M, Fermanian J, Mouthon L (2007) Assessing disability and quality of life in systemic sclerosis: construct validities of the Cochin Hand Function Scale, Health Assessment Questionnaire (HAQ), Systemic Sclerosis HAQ, and Medical Outcomes Study 36-Item Short Form Health Survey. Arthritis Rheum 57:94–102. https://doi.org/10.1002/art.22468
Thom DH, Haan MN, Van Den Eeden SK (1997) Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing 26:367–374. https://doi.org/10.1093/ageing/26.5.367
Buckley BS, Lapitan MC (2010) Prevalence of urinary incontinence in men, women, and children–current evidence: findings of the Fourth International Consultation on Incontinence. Urology 76:265–270. https://doi.org/10.1016/j.urology.2009.11.078
Minassian VA, Bazi T, Stewart WF (2017) Clinical epidemiological insights into urinary incontinence. Int Urogynecol J 28:687–696. https://doi.org/10.1007/s00192-017-3314-7
Minassian VA, Drutz HP, Al-Badr A (2003) Urinary incontinence as a worldwide problem. Int J Gynaecol Obstet 82:327–338. https://doi.org/10.1016/s0020-7292(03)00220-0
Ebbesen MH, Hunskaar S, Rortveit G, Hannestad YS (2013) Prevalence, incidence and remission of urinary incontinence in women: longitudinal data from the Norwegian HUNT study (EPINCONT). BMC Urol 13:27. https://doi.org/10.1186/1471-2490-13-27
Komesu YM, Schrader RM, Ketai LH, Rogers RG, Dunivan GC (2016) Epidemiology of mixed, stress, and urgency urinary incontinence in middle-aged/older women: the importance of incontinence history. Int Urogynecol J 27:763–772. https://doi.org/10.1007/s00192-015-2888-1
Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P (2004) ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 23:322–330. https://doi.org/10.1002/nau.20041
Espuna Pons M, Rebollo Alvarez P, Puig Clota M (2004) Validation of the Spanish version of the International Consultation on Incontinence Questionnaire-Short Form. A questionnaire for assessing the urinary incontinence. Med Clin (Barc) 122:288–292. https://doi.org/10.1016/s0025-7753(04)74212-8
Funding
Open access funding provided by University of Geneva. This research received no financial support from any private or public authorities.
Author information
Authors and Affiliations
Contributions
Each author fulfils the ICMJE’ conditions for authorship and attests that they have directly participated in the preparation of this manuscript and that they have read and approved the final version submitted. All authors have participated in conception and design, or data analysis and interpretation, drafting the article or revising it critically for important intellectual content.
CRediT authors statement GJ: Conceptualization (lead), formal analysis (lead), data curation (lead), writing original draft (lead); EZ: Investigation (equal), data curation (supporting), writing review & editing (equal); PP: Investigation (equal), writing review & editing (equal); DMS: Investigation (equal), writing review & editing (equal); PP: Investigation (equal), writing review & editing (equal); SP: Investigation (equal), writing review & editing (equal); MF: Investigation (equal), writing review & editing (equal); YC: Investigation (equal), writing review & editing (equal); RG: Investigation (equal), writing review & editing (equal); FF: Conceptualization (supporting), writing original draft (supporting), writing review & editing (equal); MET: Conceptualization (supporting), writing original draft (supporting), writing review & editing (equal); FC: Conceptualization (supporting), writing original draft (supporting), writing review & editing (equal); PA: Conceptualization (supporting), writing original draft (supporting), writing review & editing (equal); CC: Conceptualization (supporting), writing original draft (supporting), writing review & editing (equal).
Corresponding author
Ethics declarations
Competing interests
All the authors have completed the Unified Competing Interest form and declare no support from any organisation for the submitted work, no financial relationships with any organisations that might have had an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Ethics
The study complies with the Declaration of Helsinki. Locally appointed ethics committee has approved the research protocol. Written informed consent has been obtained from all patients included in the study.
-
Geneva: Approving institution: Comission d’éthique de la recherché sur l’être humain (CEREH); Decision number: CER:12–291; decision date: the 15.th of January 2013
-
Bordeaux: Approving institution: Comité De Protection Des Personnes Sud-Ouest Et Outre Mer III; Decision number: DC 2014/29; decision date: the 18.th of April 2014
-
Brescia: Approving institution: Spedali Civili-Brescia Azienda Ospedaliera Comitato Etico; Decision number: NP n.1410; decision date: the 12.th of July 2013
-
Padua: Approving institution: Azienda Ospedaliera E Universita’ Degli Studi Di Padova, Comitato Etico Per La Sperimentazione; Decision number: Prot. n.2979; decision date: the 8.th of July 2013
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
John, G., Zanatta, E., Polito, P. et al. Urinary incontinence in systemic sclerosis: a prospective multicentre cohort study. Rheumatol Int 42, 2141–2150 (2022). https://doi.org/10.1007/s00296-022-05178-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05178-1