Abstract
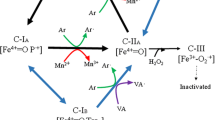
Previously, we suppressed the expression of genes encoding isozymes of lignin peroxidase (LiP) and manganese peroxidase (MnP) using a calmodulin (CaM) inhibitor, W7, in the white-rot fungus Phanerochaete chrysosporium; this suggested that CaM positively regulates their expression. Here, we studied the role of CaM in another white-rot fungus, Pleurotus ostreatus, which produces MnP and versatile peroxidase (VP), but not LiP. W7 upregulated Mn2+-dependent oxidation of guaiacol, suggesting that CaM negatively regulates the production of the enzymes. Suppression of CaM in P. ostreatus using RNAi also led to upregulation of enzyme activity, whereas overexpression of CaM in P. ostreatus caused downregulation. Real-time RT-PCR showed that MnP1-6 and VP3 levels in the CaM-knockdown strain were higher than those in the wild-type strain, while MnP-5 and -6 and VP1 and 2 levels in the CaM-overexpressing strain were lower than in the wild type. Moreover, we also found that another ligninolytic enzyme, laccase, which is not produced by P. chrysosporium, was negatively regulated by CaM in P. ostreatus similar to MnP and VP. Although overexpression of CaM did not reduce the ability of P. ostreatus to digest beech wood powder, the percentage of lignin remaining in the digest was slightly higher than in the wild-type strain digest.












Similar content being viewed by others
References
Ahn I-P, Suh S-C (2007) Calcium/calmodulin-dependent signaling for prepenetration development in Cochliobolus miyabeanus infecting rice. J Gen Plant Pathol 73:113–120
Ahn I-P, Uhm K-H, Kim S, Lee Y-H (2003) Signaling pathways involved in preinfection development of Colletotrichum gloeosporioides, C. coccodes, and C. dematium pathogenic on red pepper. Physiol Mol Plant Pathol 63:281–289
Amore A, Honda Y, Faraco V (2012) Copper induction of enhanced green fluorescent protein expression in Pleurotus ostreatus driven by laccase poxa1b promoter. FEMS Microbiol Lett 337:155–163
Belinky PA, Flikshtein N, Lechenko S, Gepstein S, Dosoretz CG (2003) Reactive oxygen species and induction of lignin peroxidase in Phanerochaete chrysosporium. Appl Environ Microbiol 69:6500–6506
Bonnarme P, Jeffries TW (1990) Mn(II) regulation of lignin peroxidases and manganese-dependent peroxidases from lignin-degrading white rot fungi. Appl Environ Microbiol 56:210–217
Boominathan K, Reddy CA (1992) cAMP-mediated differential regulation of lignin peroxidase and manganese-dependent peroxidase production in the white-rot basidiomycete Phanerochaete chrysosporium. Proc Natl Acad Sci USA 89:5586–5590
Brown JA, Glenn JK, Gold MH (1990) Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol 172:3125–3130
Choinowski T, Blodig W, Winterhalter KH, Piontek K (1999) The crystal structure of lignin peroxidase at 1.70 Å resolution reveals a hydroxy group on the Cβ of tryptophan 171: a novel radical site formed during the redox cycle. J Mol Biol 286:809–827
Cullen D, Kersten PJ (2004) Enzymology and molecular biology of lignin degradation. In: Brambl R, Marzluf GA (eds) Biochemistry and molecular biology (The Mycota). Springer, New York, pp 249–273
Dashtban M, Schraft H, Syed TA, Qin W (2010) Fungal biodegradation and enzymatic modification of lignin. Int J Biochem Mol Biol 1:36–50
Davis TN, Urdea MS, Masiarz FR, Thorner J (1986) Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell 47:423–431
Dence CW (1992) The determination of lignin. In: Lin SY, Dence CW (eds) Methods in lignin chemistry. Springer, Heidelberg, pp 33–61
Fernandez-Fueyo E, Castanera R, Ruiz-Dueñas FJ, Lopez-Lucendo MF, Ramirez L, Pisabarro AG, Martinez AT (2014a) Ligninolytic peroxidase gene expression by Pleurotus ostreatus: differential regulation in lignocellulose medium and effect of temperature and pH. Fungal Genet Biol 18:003
Fernandez-Fueyo E, Ruiz-Dueñas FJ, Martinez MJ, Romero A, Hammel KE, Medrano FJ, Martinez AT (2014b) Ligninolytic peroxidase genes in the oyster mushroom genome: heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability. Biotechnol Biofuels 7:2
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TK, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Dueñas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Gold MH, Kuwahara M, Chiu AA, Glenn JK (1984) Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys 234:353–362
Hatfielda R, Fukushima RS (2005) Can lignin be accurately measured? Crop Sci 45:832–839
Heinzkill M, Messner K (1997) The ligninolytic system of fungi. In: Anke T (ed) Fungal biotechnology. Chapman & Hall, London, pp 213–227
Hidaka H, Sasaki Y, Tanaka T, Endo T, Ohno S, Fujii Y, Nagata T (1981) N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci USA 78:4354–4357
Hoeflich KP, Ikura M (2002) Calmodulin in action: diversity in target recognition and activation mechanisms. Cell 108:739–742
Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T (2010) New and classic families of secreted fungal heme peroxidases. Appl Microbiol Biotechnol 87:871–897
Honda Y, Matsuyama T, Irie T, Watanabe T, Kuwahara M (2000) Carboxin resistance transformation of the homobasidiomycete fungus Pleurotus ostreatus. Curr Genet 37:209–212
Irie T, Honda Y, Hirano T, Sato T, Enei H, Watanabe T, Kuwahara M (2001a) Stable transformation of Pleurotus ostreatus to hygromycin B resistance using Lentinus edodes GPD expression signals. Appl Microbiol Biotechnol 56:707–709
Irie T, Honda Y, Watanabe T, Kuwahara M (2001b) Efficient transformation of filamentous fungus Pleurotus ostreatus using single-strand carrier DNA. Appl Microbiol Biotechnol 55:563–565
Irie T, Honda Y, Watanabe T, Kuwahara M (2001c) Homologous expression of recombinant manganese peroxidase genes in ligninolytic fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 55:566–570
Kerem Z, Hadar Y (1993) Effect of manganese on lignin degradation by Pleurotus ostreatus during solid-state fermentation. Appl Environ Microbiol 59:4115–4120
Kirk TK, Connors WJ, Bleam RD, Hackett WF, Zeikus JG (1975) Preparation and microbial decomposition of synthetic C14 ligins. Proc Natl Acad Sci USA 72:2515–2519
Kobayashi I, Yamada M, Kobayashi Y (2007) Calcium ion promotes successful penetration of powdery mildew fungi into barley cells. J Gen Plant Pathol 73:399–404
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Li D, AIic M, Brown JA, Gold MH (1995) Regulation of manganese peroxidase gene transcription by hydrogen peroxide, chemical stress, and molecular oxygen. Appl Environ Microbiol 61:341–345
MacDonald MJ, Paterson A, Broda P (1984) Possible relationship between cyclic AMP and idiophasic metabolism in the white rot fungus Phanerochaete chrysosporium. J Bacteriol 160:470–472
Martinez D, Larrondo LF, Putnam N, Gelpke MD, Huang K, Chapman J, Helfenbein KG, Ramaiya P, Detter JC, Larimer F, Coutinho PM, Henrissat B, Berka R, Cullen D, Rokhsar D (2004) Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol 22:695–700
Minami M, Kureha O, Mori M, Kamitsuji H, Suzuki K, Irie T (2007) Long serial analysis of gene expression for transcriptome profiling during the initiation of ligninolytic enzymes production in Phanerochaete chrysosporium. Appl Microbiol Biotechnol 75:609–618
Minami M, Suzuki K, Shimizu A, Hongo T, Sakamoto T, Ohyama N, Kitaura H, Kusaka A, Iwama K, Irie T (2009) Changes in the gene expression of the white rot fungus Phanerochaete chrysosporium due to the addition of a tropine. Biosci Biotechnol Biochem 73:1722–1731
Morales M, Mate MJ, Romero A, Martinez MJ, Martinez AT, Ruiz-Dueñas FJ (2012) Two oxidation sites for low redox potential substrates: a directed mutagenesis, kinetic, and crystallographic study on Pleurotus eryngii versatile peroxidase. J Biol Chem 287:41053–41067
Osawa M, Swindells MB, Tanikawa J, Tanaka T, Mase T, Furuya T, Ikura M (1998) Solution structure of calmodulin-W-7 complex: the basis of diversity in molecular recognition. J Mol Biol 276:165–176
Palmieri G, Giardina P, Marzullo L, Desiderio B, Nitti G, Cannio R, Sannia G (1993) Stability and activity of a phenol oxidase from the ligninolytic fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 39:632–636
Paszczynski A, Pasti-Grigsby MB, Goszczynski S, Crawford RL, Crawford DL (1992) Mineralization of sulfonated azo dyes and sulfanilic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl Environ Microbiol 58:3598–3604
Pezzella C, Autore F, Giardina P, Piscitelli A, Sannia G, Faraco V (2009) The Pleurotus ostreatus laccase multi-gene family: isolation and heterologous expression of new family members. Curr Genet 55:45–57
Pezzella C, Lettera V, Piscitelli A, Giardina P, Sannia G (2013) Transcriptional analysis of Pleurotus ostreatus laccase genes. Appl Microbiol Biotechnol 97:705–717
Ruiz-Dueñas FJ, Morales M, Perez-Boada M, Choinowski T, Martínez MJ, Piontek K, Martínez AT (2007) Manganese oxidation site in Pleurotus eryngii versatile peroxidase: a site-directed mutagenesis, kinetic, and crystallographic study. Biochemistry 46:66–77
Ruiz-Dueñas FJ, Fernández E, Martínez MJ, Martínez AT (2011) Pleurotus ostreatus heme peroxidases: an in silico analysis from the genome sequence to the enzyme molecular structure. C R Biol 334:795–805
Sakamoto T, Kitaura H, Minami M, Honda Y, Watanabe T, Ueda A, Suzuki K, Irie T (2010) Transcriptional effect of a calmodulin inhibitor, W-7, on the ligninolytic enzyme genes in Phanerochaete chrysosporium. Curr Genet 56:401–410
Sakamoto T, Yao Y, Hida Y, Honda Y, Watanabe T, Hashigaya W, Suzuki K, Irie T (2012) A calmodulin inhibitor, W-7 influences the effect of cyclic adenosine 3′, 5′-monophosphate signaling on ligninolytic enzyme gene expression in Phanerochaete chrysosporium. AMB Express 2:7
Sakamoto T, Honda Y, Kameshita I, Suzuki K, Irie T (2013) Isolation and heterologous expression of the Phanerochaete chrysosporium calmodulin gene. Mycoscience 54:241–246
Salame TM, Yarden O, Hadar Y (2010) Pleurotus ostreatus manganese-dependent peroxidase silencing impairs decolourization of Orange II. Microb Biotechnol 3:93–106
Salame TM, Knop D, Tal D, Levinson D, Yarden O, Hadar Y (2012) Predominance of a versatile-peroxidase-encoding gene, mnp4, as demonstrated by gene replacement via a gene targeting system for Pleurotus ostreatus. Appl Environ Microbiol 78:5341–5352
Salame TM, Knop D, Levinson D, Yarden O, Hadar Y (2013) Redundancy among manganese peroxidases in Pleurotus ostreatus. Appl Environ Microbiol 79:2405–2415
Salame TM, Knop D, Levinson D, Mabjeesh SJ, Yarden O, Hadar Y (2014) Inactivation of a Pleurotus ostreatus versatile peroxidase-encoding gene (mnp2) results in reduced lignin degradation. Environ Microbiol 16:225–277
Sato T, Ueno Y, Watanabe T, Mikami T, Matsumoto T (2004) Role of Ca2+/calmodulin signaling pathway on morphological development of Candida albicans. Biol Pharm Bull 27:1281–1284
Singh D, Zeng J, Chen S (2011) Increasing manganese peroxidase productivity of Phanerochaete chrysosporium by optimizing carbon sources and supplementing small molecules. Lett Appl Microbiol 53:120–123
Wang G, Lu L, Zhang CY, Singapuri A, Yuan S (2006) Calmodulin concentrates at the apex of growing hyphae and localizes to the Spitzenkörper in Aspergillus nidulans. Protoplasma 228:159–166
Yao Y, Sakamoto T, Honda Y, Kagotani Y, Izumitsu K, Suzuki K, Irie T (2013) The white-rot fungus Pleurotus ostreatus transformant overproduced intracellular cAMP and laccase. Biosci Biotechnol Biochem 77:2309–2311
Acknowledgments
This work was partially supported by the grant of an FS Stage Project for Advanced Low Carbon Technology Research and the Development Program of the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Kües.
Rights and permissions
About this article
Cite this article
Suetomi, T., Sakamoto, T., Tokunaga, Y. et al. Effects of calmodulin on expression of lignin-modifying enzymes in Pleurotus ostreatus . Curr Genet 61, 127–140 (2015). https://doi.org/10.1007/s00294-014-0460-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-014-0460-z