Abstract
In this study, poly(ɛ-caprolactone-g-2-hydroxy ethyl methacrylate) [P(CL-g-HEMA)] graft copolymer hydrogels were successfully synthesized through multi-step reactions. For this purpose, firstly, hydroxyl-terminated poly(ɛ-caprolactone) (PCL-OH) was obtained by ring-opening polymerization (ROP) method of ɛ-caprolactone using 3-chlor-1,2-propanediol initiator, which is suitable for ring-opening polymerization method. Then, from the reaction of synthesized PCL-OH and 3-bromopropionyl chloride, a new brominated poly(ɛ-caprolactone) (PCL-Br) was synthesized for use as a functionalized atom transfer radical polymerization (ATRP) initiator. Poly(ɛ-caprolactone-g-2-hydroxy ethyl methacrylate) [P(CL-g-HEMA)] graft copolymer hydrogels were synthesized by “grafting from” atom transfer radical polymerization (ATRP) of 2-hydroxy ethyl methacrylate (HEMA) presence the new synthesized functionalized ATRP initiator (PCL-Br) and hydrogel properties were investigated. The synthesized functionalized initiators and graft copolymer hydrogel were characterized by spectroscopic methods such as 1H-NMR, FT-IR, TGA, DSC and SEM. The observation of two different decomposition temperatures, respectively, from the TGA analysis results may support the formation of the biblock graft copolymer. A glass transition temperature (Tg) of the graft copolymer hydrogel was found by DSC, and this value is between the Tg values of the homopolymers forming the graft copolymer hydrogel. Water swelling values of graft copolymer hydrogels were measured and calculated every 24 h in pure water with pH = 7 at from + 4 to 65 °C. Considering the weight of dry graft copolymer hydrogels, it was seen that water was absorbed at most at + 4 °C. As the temperature increased, the water absorption or swelling of the hydrogel decreased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graft copolymers have been extensively studied for their intriguing properties that are inherently different compared to their linear counterparts, highlighting their importance as a macromolecular structure. For example, in graft polymers where branches are grafted very densely onto the polymer backbone, steric interactions between side chains cause partial or complete elongation of the backbone, resulting in unusual rheological behavior [1,2,3,4,5,6,7]. This has led to a number of applications of graft copolymers that exploit their mechanical and viscoelastic properties. The preparation methods of such polymers are interesting because the properties of graft copolymers are quite different from those of the homopolymer of the monomers from which they are formed. Recently, starting from controlled polymerization processes, macromolecules with complex architecture, in which polymer end-of-chain functionality or graft chain part is formed, are mostly synthesized [8,9,10,11,12,13,14]. Well-defined graft copolymers are often difficult to synthesize due to their complex and limited structure compared to their linear counterparts [15]. In general, three different strategies can be used to synthesize graft copolymers, such as “grafting through,” “grafting from” and “grafting to.” Through living/controlled radical polymerization processes, well-defined graft copolymers can often be prepared by either a “grafting through” or a “grafting from” strategy [15,16,17]. However, a third idea based on “grafting to” chemistry has been proposed with the introduction of “click” chemistry [18]. With the advent of living/controlled radical polymerization techniques, the “grafting from” strategy (polymerization of a second monomer on active centers on a polymeric backbone) is a more convenient and therefore more preferred synthesis method compared to the “grafting through” and “grafting to” strategies. On the other hand, the structure needs to be chemically modified to attach active functional groups to the macromolecular backbone, but it is difficult to regulate the number of grafting sites due to the complexity of the macromolecular reaction [15, 19, 20].
Polymers of desired molecular weight and well-defined can be prepared by controlled radical polymerization methods. These methods are nitroxide-centered polymerization (NMP) [21], atom transfer radical polymerization (ATRP) [22] and reversible addition/fragmentation chain transfer (RAFT) polymerization [23]. Among the controlled radical polymerization methods that have been developed continuously in recent years, the most attention has been directed to ATRP, and it has been the subject of many researches. With these methods, free-radical polymerization has been transformed into a controlled/living system, enabling the development of new polymeric materials. Compared to other living radical systems, ATRP is simpler, cheaper and more suitable for controlled radical polymerization. Matyjaszewski reported in 1995 atom transfer radical polymerization (ATRP), a type of control/living radical polymerization, using the alkyl halide and Cu(I)/ligand catalyst system as initiator [22]. In ring-opening polymerization (ROP), cyclic ethers, esters, amides, etc., are used [24,25,26].
In this study, graft copolymer hydrogels were synthesized by “grafting from” ATRP method, which is one of the controlled radical polymerization techniques, using a new bromine-terminated functionalized ATRP initiator synthesized by ring-opening polymerization (ROP). PCL is of great interest to researchers due to its potential applications and is a biodegradable aliphatic polyester. PCL and its copolymers are used as biomedical materials due to the advantage of two common melting temperatures, surgical suture, controlled drug delivery, bone fixation device, tissue repair materials, magnet and degradability rate of the most important polymers [26,27,28,29]. Poly(2-hydroxy ethyl methacrylate) (PHEMA) hydrogel is resistant to absorption by the body; it is inert to biological processes and has good permeability to metabolites. In most cases, HEMA-based copolymers have good hemocompatibility and biocompatibility, are non-toxic and hydrophilic, which is desirable for applications in medicine. Homopolymer and copolymers of HEMA can be a matrix for anticancer drugs, hormones, enzymes, antibiotics, peptides, cells and many more biologically active species [30,31,32].
In this contribution, poly(ɛ-caprolactone-g-2-hydroxy ethyl methacrylate) [P(CL-g-HEMA)] graft copolymer hydrogels were synthesized by “grafting from” atom transfer radical polymerization (ATRP) and ring-opening polymerization (ROP) methods. For this purpose, hydroxyl-terminated poly(ɛ-caprolactone) (PCL-OH) was obtained by ring-opening polymerization method of ɛ-caprolactone using 3-chlor-1,2-propanediol initiator. Then, from the reaction of synthesized PCL-OH and 3-bromopropionyl chloride, a new bromine-terminated poly(ɛ-caprolactone) (PCL-Br) was synthesized for use as a functionalized ATRP initiator. Poly(ɛ-caprolactone-g-2-hydroxy ethyl methacrylate) [P(CL-g-HEMA)] graft copolymer hydrogels were synthesized by “grafting from” ATRP of the HEMA using the new synthesized functionalized ATRP initiator and hydrogel and thermal properties were also studied.
Materials and methods
Reactants
The 2-hydroxy ethyl methacrylate (HEMA, 97%), 3-chloro-1.2-propanediol (98%), dibutyltin dilaurate (DBTDL, %95), trietilenamin (TEA, 99%), 3-bromopropionyl chloride (98%), copper(I) bromide (98%) and diethyl ether (99.5%) were received from Aldrich and used as received. Dichloromethane (99.8%), ɛ-caprolactone (ɛ-CL, 99%) were supplied from Merck. N,N,N′,N′′,N′′ pentamethyldiethylenetriamine (PMDETA, 99%) was received by Acros.
Synthesis of hydroxyl-terminated poly(ɛ-caprolactone) by ring-opening polymerization
For this synthesis, 1 g of 3-chloro-1,2-propanediol and 15 g of ɛ-caprolactone and 1–2 drops of DBDTL were placed in a 250 mL glass flask and mixed on a magnetic stirrer for 10 h in a 120 °C oil bath. Then, the contents of the balloon were dropped into cold diethyl ether and hydroxyl-terminated poly(ɛ-caprolactone) (PCL-OH) was precipitated. The product acquired was left to dry under vacuum at room temperature for one week. PCL-OH was weighed, and the yield was calculated as 92% gravimetrically. The synthesis mechanism for PCL-OH is shown in Scheme 1.
Synthesis of a new functionalized brominated ATRP (PCL-Br) initiator
10 g of PCL-OH was taken into a flask and 10 mL of dichloromethane was added as a solvent and this mixture was stirred on a magnetic stirrer in an ice bath at 0 °C for one hour. Then, 1.1629 g of triethylenediamine was added to this mixture and mixed again for one hour. In another a flask, 1.9689 g of 3-bromopropionyl chloride was taken, then 5 mL of dichloromethane was added and dissolved and this solution was added dropwise to the solution in the ice bath with the help of a separating funnel. The reaction mixture was allowed to stir at room temperature for 24 h, and precipitated in cold diethyl ether. As a result of this reaction, functionalized brominated PCL (PCL-Br) was obtained and dried under vacuum for one week. The yield was calculated by weighing the synthesized product, and the yield was found to be 42% by weight. The reaction steps are shown in the Scheme 2.
Synthesis of poly(ɛ-caprolactone-g-2-hydroxy ethyl methacrylate) graft copolymer hydrogels via “grafting from” ATRP
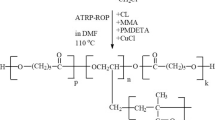
For this ATRP polymerization, certain amounts of PCL-Br, HEMA, PMDETA and CuBr were placed in a Schlenk tube and homogeneous solution formation was achieved. The weights of the chemicals used in this polymerization are given in Tables 1 and 2. The tube was tightly closed and placed in a silicone oil bath at 80 °C, and “grafting from” ATRP polymerization of the HEMA was performed. At the end of the polymerization, the tube contents were poured into cold diethyl ether and the P(CL-g-HEMA) graft copolymer hydrogels were precipitated. The obtained graft copolymer hydrogels were dried at room temperature under vacuum for one week. The polymerization reaction mechanism is shown in Scheme 3.
Swelling tests of hydrogel graft copolymers
Approximately 0.03 g of dry hydrogel graft copolymer samples (UU-2, UU-3, UU-4 and UU-5) were taken into a vial, and 10 mL of distilled water (pH = 7) was added. The hydrogel graft copolymer samples were first kept in the refrigerator to swell in water at from + 4 °C to 65 °C for 24 h. At the end of this period, the samples were weighed and the swelling values in water were weighed and recorded.
Structural and thermal analyzes
1H-NMR
1H-NMR analysis of the synthesized initiators and the graft copolymer hydrogels were performed on a Bruker Ultra Shield Plus, ultra-long retention time 400 MHz NMR spectrometer using DMSO as a solvent.
FT-IR
Fourier transform infrared spectroscopy (FT-IR) analysis was performed to determine the functional groups associated with the initiators and graft copolymer hydrogels. The spectra were recorded with a PerkinElmer (Shelton, CT, USA) Spectrum 100 spectrometer in transmit mode with a scanning speed of 4000 to 550 cm-1.
TGA/DSC
Thermal analyzes of the obtained products were performed with LABSYS EVO TGA/DSC thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) device under Argon gas with a heating rate of 10 °C/min. The TGA temperature range was from 25 °C to 550 °C while DSC was performed using a temperature range between 25 °C and 200 °C.
GPC
The molecular weights and molecular weight distributions of the synthesized product were determined by gel-permeation chromatography (GPC) on a Polymer Laboratories PL-GPC 220 and HPLC-Shimadzu; LC-20AD gel-permeation chromatography (GPC) using THF as a solvent. Each of the samples was weighed (~ 25 mg) and dissolved in 10 mL of THF. The dissolved samples were transferred to separate vials after passing each through a 0.22 PTFE syringe filter. A polystyrene (PS) calibration curve with 12 standards ranging from 1000 Da to 4.5 MDa was used for post-analysis calculations.
SEM analysis
The surface morphology of the synthesized graft copolymer hydrogels was performed on a scanning electron microscope ZEISS SIGMA 300 FESEM with an energy X-ray spectroscopy (EDX).
Results and discussion
Synthesis of hydroxyl-terminated poly(ɛ-caprolactone)
In the route of this synthesis, ɛ-caprolactone was polymerized using 3-chloro-1.2-propanediol, a suitable initiator for ring-opening polymerization (ROP), and hydroxyl-terminated poly(ɛ-caprolactone) (PCL-OH) was obtained. 3-chloro-1.2-propanediol and PCL-OH were characterized by FT-IR and 1H-NMR. In addition, the molecular weight of PCL-OH was determined by GPC. In the FT-IR spectra of 3-chloro-1.2-propanediol shown in Fig. 1a, peaks of 3330 cm−1: –OH and 2930 cm−1: Aliphatic –CH bands are seen. In the 1H-NMR scan of 3-chloro-1.2-propanediol in Fig. 2a, δ: 3.3 ppm –OH, δ:3.4 ppm –Cl bound –CH2, δ: 3.6 ppm –CH and δ:3.6 ppm –CH2 protons peaks were recorded. In the FT-IR spectra of PCL-OH in Fig. 1b showed peaks of 3330 cm−1: –OH, 2942 and 2864 cm−1 aliphatic –CH, 1721 cm−1: C = O bands. Similarly, the PCL-OH 1H-NMR scan in Fig. 2b; δ: 1.3 and 1.5 ppm PCL-OH’s –CH2, δ: 3.6 and 3.7 ppm 3-chloro-1.2-propanediol’s –CH, –CH2, δ: 3.9 ppm PCL-OH’s –OCH2 protons peaks were determined.
In the GPC diagram shown in Fig. 3, the molecular weight (MnGPC) of PCL-OH was found to be 1737 g/mol and the Mw/Mn value was 1.2. The molecular weight (MnNMR) of PCL-OH was calculated as 2385 g/mol by using the integral ratio of the –OCH2 peak seen at 3.9 ppm in the 1H-NMR spectrum shown in Fig. 2b [33, 34].
Synthesis of a new functionalized ATRP initiator (PCL-Br)
A new functionalized ATRP initiator (PCL-Br) was obtained from the reaction of PCL-OH and 3-bromopropionyl chloride. The new functionalized ATRP initiator (PCL-Br) was introduced to the literature with this study. PCL-Br was also characterized by FT-IR and 1H-NMR. In the FT-IR spectra of a new brominated ATRP initiator (PCL-Br) shown in Fig. 4a, 1722 cm−1: C = O and 2358–2940 cm−1: peaks of aliphatic –CH groups detected. In the 1H-NMR spectrum of PCL-Br shown in Fig. 5a, δ: 1.1 and 1.5 ppm of the PCL and of the 3-bromopropionyl –CH2, δ: 3.4 ppm of the 3-bromopropionyl –CH2-Br and δ: 3.9 ppm of the PCL –OCH2 peaks of the protons were detected.
Synthesis of graft copolymer hydrogels via by “grafting from” ATRP polymerization
Poly(ɛ-caprolactone-g-2-hydroxy ethyl methacrylate) (PCL-g-PHEMA) graft copolymer hydrogels were synthesized by “grafting from” atom transfer radical polymerization (ATRP) of HEMA in the presence of a novel functionalized ATRP initiator (PCL-Br). The polymerization conditions and results of the synthesized graft copolymer hydrogels are given in Tables 1 and 2. Synthesized graft copolymer hydrogels were characterized FT-IR, 1H-NMR, TGA, DSC and SEM. The FT-IR scan of the PCL-g-PHEMA graft copolymer hydrogels given in Fig. 4b, 1715 cm−1: C = O group, 2950 cm−1: aliphatic –CH group and 3400 cm−1: –OH group peaks were observed. In the 1H-NMR scan of the PCL-g-PHEMA graft copolymer hydrogels in Fig. 5b, δ: 0.7 and 0.9 ppm PHEMA block –CH3 protons, δ: 1.8 ppm PHEMA, PCL block and propionyl group, δ: OH–CH2 of 3.8 ppm PHEMA block, δ: 4.0 ppm PHEMA and PCL blocks –OCH2 and δ: 7.7 and 6.0 ppm PHEMA block –OH peaks of the protons were determined. The molecular weight (MnNMR) of PCL-g-PH EMA graft copolymer was calculated in the 1H-NMR spectrum shown in Fig. 5b, with the integral ratios of PCL’s –OCH2 at 4.0 ppm and PHEMA’s large –OH peaks at 4.7–6.0 ppm [33, 34]. The molecular weight (MnNMR) of the graft copolymer hydrogels is shown in Tables 1 and 2.
Investigation of thermal properties of graft copolymer hydrogels
The thermal properties of the synthesized PCL-g-PHEMA graft copolymer hydrogels were determined using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). Figures. 6 and 7 show the TGA and dTG (Differential TGA) diagrams of the graft copolymer hydrogel, respectively. According to the mass degradation TGA curves of the graft copolymers shown in Figs. 6 and 7, when the graft copolymer hydrogels were heated from 25 °C to 600 °C, it was observed that they completely melted with two decomposition temperatures. The decomposition temperatures shown in Figs. 6 and 7 are shown in the TGA curves, and decomposition temperatures were observed for PCL and PHEMA blocks at 205–325 °C and 410–425 °C, respectively (Tables 1 and 2).
Figure 8 shows the DSC analysis of the PCL-g-PHEMA graft copolymer hydrogel. The glass transition temperature value of 31 °C for the synthesized PCL-g-PHEMA graft copolymer hydrogel (UA-2, UU-3 and UU-4, in Tables 1 and 2) can be said to be mixed at a reasonable rate to obtain graft copolymer from homopolymers. This glass transition temperature value is between −73 °C for PCL [35] and 99.3 °C for PHEMA [36] as in the literature.
The surface morphology of the PCL-g-PHEMA graft copolymer hydrogels was scanned by scanning electron microscopy (SEM). There appears to be some agglomeration in the surface morphology shown in Fig. 9. It can be said that these agglomerations belong to PHEMA (hydrogel) and smooth surfaces belong to PCL.
Investigation of hydrogel properties of graft copolymer
The percentage swelling values of hydrogels in water were calculated using the following Eq. 1. In the following Eq. 1, the Mw value represents the wet hydrogel and the Md value represents the dry hydrogel. Based on 0.03 g dry weight, the polymer samples shown in Fig. 10 reached the maximum absorption percentage by absorbing water at maximum + 4 °C. At + 4 °C, and it was observed that UU-2 absorbed the most water, while UU-3 absorbed the least amount of water according to its dry weight. As the temperature increased, the water absorption percentage of the samples began to decrease compared to + 4 °C. While the percentage values of swelling in water remained relatively constant as the temperature increased for UU-3, UU-4 and UU-5 at 55 °C, it was observed that there was a decrease in the water swelling value for UU-2.
Conclusion
In conclusion, hydroxyl-terminated poly(ɛ-caprolactone) (PCL-OH) was synthesized by ring-opening polymerization of ɛ-caprolactone from the -OH ends of 3-chlor-1,2-propanediol. The functionalized ATRP initiator (PCL-Br) was obtained by bromination of the -OH active centers formed on the surfaces of PCL. Finally, the “grafting from” atom transfer radical polymerization (ATRP) of 2-hydroxy ethyl methacrylate (HEMA) was polymerized on the surface of the functionalized ATRP initiator (PCL-Br) and poly (ɛ-caprolactone-g-2-hydroxy ethyl methacrylate) [P(CL-g-HEMA)] graft copolymer hydrogels were obtained, and hydrogel properties investigated. Spectroscopic methods were mostly preferred for characterization. The thermal properties of the synthesized graft copolymer hydrogels were investigated using TGA and DSC analyses. Observation of two different degradation temperatures by TGA analysis may confirm the formation of a biblock graft copolymer. In the DSC analysis, one glass transition temperature (Tg) was found and this value was between the Tg values of the homopolymers forming the graft copolymer hydrogel. Swelling values of graft copolymer hydrogels in water were measured and calculated every 24 h at from + 4 °C to 65 °C in pure water with pH = 7. Considering the weight of the dry graft copolymer hydrogels, it was observed that the water was absorbed the most at + 4 °C. As the temperature increased, the water absorption or swelling of the hydrogel decreased.
Data availability
No datasets were generated or analysed during the current study.
References
Kim J, Cattoz B, Leung AHM, Parish JD, Becer CR (2022) Enabling reversible addition-fragmentation chain-transfer polymerization for brush copolymers with a poly(2-oxazoline) backbone. Macromolecules 55:4411–4419. https://doi.org/10.1021/acs.macromol.2c00497
Tanaka J, Hakkinen S, Boeck PT, Cong Y, Perrier S, Sheiko SS, You W (2020) Orthogonal cationic and radical RAFT polymerizations to prepare bottlebrush polymers. Angew Chem Int Ed 59:7203–7208. https://doi.org/10.1002/anie.202000700
Shanmugam S, Cuthbert J, Kowalewski T, Boyer C, Matyjaszewski K (2018) Catalyst-free selective photoactivation of RAFT polymerization: a facile route for preparation of comblike and bottlebrush polymers. Macromolecules 51:7776–7784. https://doi.org/10.1021/acs.macromol.8b01708
Cui Y, Jiang X, Feng C, Gu G, Xu J, Huang X (2016) First double hydrophilic graft copolymer bearing a poly(2-hydroxylethyl acrylate) backbone synthesized by sequential RAFT polymerization and SET-LRP. Polym Chem 7:3156–3164. https://doi.org/10.1039/c6py00489j
Kerr A, Hartlieb M, Sanchis J, Smith T, Perrier S (2017) Complex multiblock bottle-brush architectures by RAFT polymerization. Chem Commun 53:11901–11904. https://doi.org/10.1039/C7CC07241D
Polymeropoulos G, Zapsas G, Ntetsikas K, Bilalis P, Gnanou Y, Hadjichristidis N (2017) 50th anniversary perspective: polymers with complex architectures. Macromolecules 50:1253–1290. https://doi.org/10.1021/acs.macromol.6b02569
Aksakal S, Beyer VP, Aksakal R, Becer CR (2019) Copper mediated RDRP of thioacrylates and their combination with acrylates and acrylamides. Polym Chem 10:6622–6629. https://doi.org/10.1039/C9PY01518C
Hazer B, Subramaniyan S, Zhang B (2021) RAFT polymerization of the novel methacrylated methyl salicylate. Block copolymerization with poly (3-hydroxy butyrate). ChemistrySelect 6:12255–12265. https://doi.org/10.1002/slct.202102977
Göktaş M (2020) Synthesis and characterization of temperature-responsive block copolymers using macromonomeric initiator. Chem Pap 74:2297–2307. https://doi.org/10.1007/s11696-020-01074-9
Hazer B (2023) Macro peroxide initiators based on autoxidized unsaturated plant oils: Block/graft copolymer conjugates for nanotechnology and biomedical applications. J Am Oil Chem Soc 100:507–520. https://doi.org/10.1002/aocs.12710
Metze FK, Filipucci I, Klok H-A (2023) Supramolecular polymer brushes grown by surface-initiated atom transfer radical polymerization from Cucurbit[7]uril-based non- covalent initiators. Angew Chem Int Ed e202305930:1–7. https://doi.org/10.1002/anie.202305930
Pal A, Karmakar PD (2023) Synthesis of copolymeric micelle (PEG-b-pMMA) through ATRP method for pH-triggered sustained release behaviour. Poly Bull. https://doi.org/10.1007/s00289-023-04914-2
Hazer B, Modjinou T, Langlois V, Göktaş M, Taşçı F, Ashby RD, Zhang B (2023) Free radical polymerization of dimethyl amino ethyl methacrylate ınitiated by Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) macroazo ınitiator: thermal and physicochemical characterization. J Polym Environ 31:3688–3699. https://doi.org/10.1007/s10924-023-02857-3
Yuan W, Yuan J, Zhou L, Wu S, Hong X (2010) Fe3O4@poly(2-hydroxyethyl methacrylate)-graft-poly(3-caprolactone) magnetic nanoparticles with branched brush polymeric shell. Polymer 51:2540–2547. https://doi.org/10.1016/j.polymer.2010.04.016
Jiang X, Jiang X, Lu G, Feng C, Huang X (2014) The first amphiphilic graft copolymer bearing a hydrophilic poly(2-hydroxylethyl acrylate) backbone synthesized by successive RAFT and ATRP. Polym Chem 5:915–4925
Kaneyoshi H, Matyjaszewski K (2006) Synthesis of a linear polyethylene macromonomer and preparation of polystyrene-graft-polyethylene copolymers via grafting-through atom transfer radical polymerization. J Appl Polym Sci 105:3–13. https://doi.org/10.1002/app.26048
Concilio M, Nguyen N, Hall CLS, Huband S, Becer CR (2023) Synthesis of Oxazoline/Methacrylate-based graft-copolymers via grafting-through method and evaluation of their self-assembly in water and dodecane. Macromolecules 56:7961–7972. https://doi.org/10.1021/acs.macromol.3c01308
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40:2004–2021. https://doi.org/10.1002/1521-3773(20010601)40:11%3c2004::aid-anie2004%3e3.3.co;2-x
Coudane J, Nottelet B, Mouton J, Garric X, Van Den Berghe H (2022) Poly(ε-caprolactone)-based graft copolymers: synthesis methods and applications in the biomedical field: a review. Molecules 27:7339. https://doi.org/10.3390/molecules27217339
Xu FJ, Wang ZH, Yang WT (2010) Surface functionalization of polycaprolactone films via surface-initiated atom transfer radical polymerization for covalently coupling cell-adhesive biomolecules. Biomaterials 31:3139–3147. https://doi.org/10.1016/j.biomaterials.2010.01.032
Maric M (2021) History of nitroxide mediated polymerization in Canada. Can J Chem Eng 99:832–852. https://doi.org/10.1002/cjce.23989
Wang JS, Matyjaszewski K (1995) Controlled/“living” radical polymerization. halogen atom transfer radical polymerization promoted by a Cu(I)/Cu(II) redox process. Macromolecules 28:7901–7910. https://doi.org/10.1021/ma00127a042
Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH (1998) Living free-radical polymerization by reversible addition – fragmentation chain transfer. The RAFT process. Macromolecules 31:5559–5562. https://doi.org/10.1021/ma9804951
Göktaş M, Öztürk T, Atalar MN, Tekeş AT, Hazer B (2014) One-step synthesis of triblock copolymers via simultaneous reversible-addition fragmentation chain transfer (RAFT) and ring-opening polymerization using a novel difunctional macro-RAFT agent based on polyethylene glycol. J Macromol Sci A: Pure Appl Chem 51:854–863. https://doi.org/10.1080/10601325.2014.953366
Göktaş M, Olgun B (2019) One-step synthesis and characterization of poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) thermo-responsive block copolymers via RAFT and ROP techniques. Polym Sci Ser B 61:421–429. https://doi.org/10.1134/s1560090419040055
Göktaş M, Aykaç C, Akinay Y (2023) Synthesis and characterization of block copolymer: thermal and morphological properties of SiO2-filled block copolymer nanocomposites. Polym Bull 80:8565–8584. https://doi.org/10.1007/s00289-022-04468-9
Göktaş M, Aykaç C, Öztürk T (2022) One-step synthesis and characterization of the block-graft terpolymer via simultaneous atom transfer radical polymerization (ATRP) and ring-opening polymerization (ROP) techniques. J Chem Sci 134:73. https://doi.org/10.1007/s12039-022-02068-8
Hu W, Safari M, Zhou Y, Pérez-Camargo RA, Liu G, Müller AJ, Wang D (2023) Comonomer inclusion in single crystals of isodimorphic random copolymers of butylene succinate and ε-caprolactone. Macromolecules 56:5058–5067. https://doi.org/10.1021/acs.macromol.3c00404
Cheechana N, Akkravijitkul N, Khamto N, Rithchumpon P, Junpirom T, Limwanich W, Punyodom W, Tantirungrotechai Y, Nimmanpipug P, Meepowpan P (2023) Room-temperature Lewis acid organocatalysts for bulk ring-opening polymerization of Bis-lithium N-heterocyclic carbenes complexes activated on ϵ-caprolactone: synthetic, experimental, and density functional theory of mechanistic studies. Macromol Chem Phys 2300129:1–8. https://doi.org/10.1002/macp.202300129
Maciej K, Daria L, Łukasz O, Andrzej D, Barbara T (2021) HEMA in Polymers with thermoresponsive properties. Polym Rev 61:714–735. https://doi.org/10.1080/15583724.2021.1896542
Passos MF, Dias DRC, Bastos GNT, Jardini AL, Benatti ACB, Dias CGBT, Maciel Filho R (2016) pHEMA hydrogels. J Therm Anal Calorim 125:361–368. https://doi.org/10.1007/s10973-016-5329-6
Yasir M, Matyjaszewski K (2023) Highly swollen ROMP-based gels. Eur Polym J 196:112295. https://doi.org/10.1016/j.eurpolymj.2023.112295
Izunobi JU, Higginbotham CL (2011) Polymer molecular weight analysis by 1H NMR spectroscopy. J Chem Educ 88:1098–1104. https://doi.org/10.1021/ed100461v
Binoy M, De P (2013) RAFT polymerization of fatty acid containing monomers: controlled synthesis of polymers from renewable resources. RSC Adv 3:24983–24990. https://doi.org/10.1039/C3RA45541F
Öztürk T, Yavuz M, Göktaş M, Hazer B (2016) One-step synthesis of triarm block copolymers by simultaneous atom transfer radical and ring-opening polymerization. Polym Bull 73:1497–1513. https://doi.org/10.1007/s00289-015-1558-2
Erol İ, Yurdakal S, Demirelli K, Gürler Z (2022) Preparation of PHEMA/TiO2 nanocomposites by combination of in-situ polymerization/hydrothermal method and determination of their thermal, swelling, biological and dielectric properties. J Polym Res 29:281. https://doi.org/10.1007/s10965-022-03146-8
Acknowledgements
This work was supported by the Yuzuncu Yil University Scientific Research Fund (grand number: FYL-2022-10290).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Melahat Göktaş took part in supervision and conceptualization. Ümran Aslan helped with conceptualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Göktaş, M., Aslan, Ü. Synthesis and characterization of graft copolymer hydrogel by “grafting from” atom transfer radical polymerization using brominated macro monomeric initiator and investigation of hydrogel properties. Polym. Bull. 81, 11127–11143 (2024). https://doi.org/10.1007/s00289-024-05241-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-024-05241-w