Abstract
A novel dithieno[3,2-b:2',3'-d]pyrrole (DTP) derivative, namely, 4-(4-(1H-pyrrol-1-yl)phenyl)-4H-dithieno[3,2-b:2',3'-d]pyrrole (DTP-Ph-Pyr) was synthesized, and its corresponding polymer (P(DTP-Ph-Pyr)) was successfully obtained via electrochemical polymerization. Electrochemical and optical properties of this novel polymer were discussed in detail. It was found that the polymer film also exhibited a reversible electrochromic behavior (orange color in the neutral state and blue color in the oxidized state) with a high optical contrast (52.5% at 950 nm) and coloration efficiency (123 cm2/C at 950 nm). Moreover, the electrochemical and optical properties of the polymer were compared with both its electrochromic P(DTP) analogues and its sister polymer structure, poly(1-(4-(1H-pyrrol-1-yl)phenyl)-2,5-di(thiophen-2-yl)-1H-pyrrole) (P(SNS-Ph-Pyr)), possessing same subunit.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is an undeniable fact that the interest in the applications of conjugated polymers has been increasing day by day [1]. Some of conjugated polymers in electronic devices have been used for the purpose of energy storage, energy trapping, energy translation, sensors, and flexible electronics [2, 3]. Depending on the emerging scientific developments, scientists are making efforts to design materials or devices with superior properties. Syntheses of conjugated polymers with different functional moieties are generally used in order to enhance their electrochemical and optical properties. Conjugated polymers have been used in applications such as solar cells [4, 5], light emitting devices [6, 7], capacitors [8, 9], electrochromic devices [10, 11], biosensors [12,13,14] and etc. Some conjugated polymers change their colors compliantly during charge insertion and extraction when they are used as integrated piece of that kind of applications as current collectors [15, 16]. When the color change occurred in the visible region, electrochromic description can be defined as coloring/bleaching states depending on electron/charge transfer activity in the polymer chain. For performances comparisons of different electrochromic conjugated polymers, scientists use some important key parameters (coloration efficiency (CE), optical contrast (ΔT %) and switching time (tsw)). There are many various conjugated monomers in the literature that are suitable for polymerization and exhibit electrochromic properties when polymerized. For example, monomers fluorene, pyrrole, thiophene, furan, carbazole, aniline are the most popular conjugated structures used for this purpose. Additionally, these structures can easily be functionalized with different subunits from their active ends [16,17,18,19,20]. Among them, thiophene and pyrrole are most commonly used conjugated monomers for electrochromic applications. However, there are some efforts in order to improve their conductivity and functionality when they are synthesized with different subunits [21, 22]. One of the solutions is to obtain the trimeric structure (2,5-di(thiophen-2-yl)-1H-pyrrole (SNS) by connecting two alpha positions of pyrrole with one alpha position of two thiophenes. In addition to the possibility of functionalizing the desired subunit from the active N terminus of the pyrrole unit located in the middle of the SNS structure, it is seen that the blocking thiophenes located on both sides of the SNS trimeric structure have alpha ends suitable for polymerization/copolymerization. In literature, there are many scientific studies related with electrochromic polymers of P(SNS) in order to provide further structure–property relationship in particular manner [23]. For example, our group was successfully synthesized a new P(SNS) electrochromic polymer (poly(1-(4-(1H-pyrrol-1-yl)phenyl)-2,5-di(thiophen-2-yl)-1H-pyrrole) (P(SNS-Ph-Pyr)) and investigated its electrochemical and optical properties [24]. The latter solution was to form a fused-ring structure of two thiophene ring with one pyrrole unit, which is called as dithieno[3,2-b:2',3'-d]pyrrole (DTP). New derivatives of DTP-based polymers are promising since DTP can be functionalized in the C2, C3 and N positions with different subunits. While several DTP-based polymers have been actively used in OFET, OPV, and OLED applications and studied in detail, there are very few studies that have investigated the electrochromic properties of N-functional DTP polymers in detail [25]. Therefore, more effort can be put on the electrochromic properties of these kind of polymer structures [25,26,27,28,29,30,31,32,33]. For whatever reason, perhaps it is due to the easier synthesis and electrochemical polymerization of the functional SNS structure, there are more studies on this subject in the literature [23]. However, DTP monomer, as donor unit, oxidizes and polymerizes more easily than the SNS structure [23, 24]. Moreover, DTP structure as donor unit triggers higher conjugation chemical structure leading to a bathochromic shift of absorption maximum and mobility of charge with lower band gap polymer formations [34,35,36,37,38].
To the best of our knowledge, in the studies carried out so far, it has not been found that the electrochemical and optical properties of two different and popular conjugated structures (trimeric SNS and fused DTP possessing the same functional group) have been compared (Scheme 1). In this study, a new DTP monomer (4-(4-(1H-pyrrol-1-yl)phenyl)-4H-dithieno[3,2-b:2',3'-d]pyrrole (DTP-Ph-Pyr) was synthesized, and its corresponding polymer P(DTP-Ph-Pyr) was electrochemically obtained. Its electrochemical and optical properties were discussed and compared with its similar analogues. In addition, the electrochromic properties of the polymer were also analyzed in detail.
Materials and method
All chemicals were purchased from Aldrich Chemical and used without purification. Polymer, poly(4-(4-(1H-pyrrol-1-yl)phenyl)-4H-dithieno[3,2-b:2',3'-d]pyrrole) (P(DTP-Ph-Pyr)) was electrochemically polymerized via cyclic voltammetry (CV) method. Three electrode systems were used during electropolymerization, electrochemical and spectroelectrochemical characterizations. For electrochemical analysis, a Pt disc (area: 0.02 cm2), Pt wire and Ag/AgCl in 3 M NaCl (aq) solution were used as working, counter, and reference electrodes, respectively. The reference electrode was calibrated using an internal ferrocene standard 5 mM solution of ferrocene at the end of every measurement. Differently, an indium tin oxide (ITO) coated glassy electrode as working electrode and Ag wire as pseudo-reference electrode (calibrated according to ferrocene) were used besides counter electrode for spectroelectrochemical studies. 0.1 M tetrabutyl ammoniumhexafluorophosphate (TBAPF6) as organic electrolytic salt and dichloromethane (DCM) and acetonitrile (ACN) as solvent were used during electrochemical, spectroelectrochemical purposes. In all experiments except scan rate dependency experiments, generally 100 mV s−1 scan rate was chosen for comparison reasons. 5 mg DTP-Ph-Pyr was dissolved in 3 ml 0.1 M TBAPF6/DCM and electropolymerization procedure was carried out. For electrochemical, spectroelectrochemical characterizations, 0.1 M TBAPF6/ACN medium was selected. Potentiostat–galvanostat studies were performed with a brand of Ivium Compactstat potentiostat galvanostat. NMR and FTIR analysis were recorded on a Bruker NMR spectrometer and Bruker Equinox 55 with an attenuated total reflectance (ATR) spectrophotometer. Spectroelectrochemical characterizations were studied via a Specord S600 UV–Vis spectrometer. Emission data were recorded with Varian Cary Eclipse Fluorescence spectrophotometer. The color analysis was also obtained by using Specord S600 UV–Vis spectrometer software.
Synthesis
Synthesis of the monomer took place in two stages as seen in Scheme 2. First, aniline-based molecule, 4-(1H-pyrrol-1-yl) aniline (1), was synthesized and then DTP-based monomer, 4-(4-(1H-pyrrol-1-yl)phenyl)-4H-dithieno[3,2-b:2',3'-d]pyrrole (DTP-Ph-Pyr), was synthesized under Pd catalysis.
Initially, 4-(1H-pyrrol-1-yl)aniline (1) was synthesized by using modified literature procedure [39]. When 4-iodoaniline was allowed to react with pyrrole in the presence of CuI, K2CO3 and dimethylethylenediamine (DMEDA) in DMF under argon, desired product was isolated in 50% yields. Then, the reaction between 3,3'-dibromo-2,2'-bithiophene and 4-(1H-pyrrol-1-yl) aniline in the presence of BINAP as catalyst and NaOtBu as a base for overnight at 110 °C gave the DTP-Ph-Pyr (21% yields).
Synthesis of 4-(1H-pyrrol-1-yl) aniline (1)
This compound was synthesized according to reference [39]. 4-Iodoaniline (1000 mg, 4.5 mmol) was stirred in DMF (10 mL) under argon gas K2CO3 (2.131 g, 10 mmol), CuI (174 mg, 9.1 mmol), dimethylethylenediamine (DMEDA) (321 mg, 3.6 mmol) and pyrrole (612 mg, 9.1 mmol) were added sequentially. The mixture was stirred at 110 °C for 24 h. Then 20 mL of distilled water was added, and the water phase was washed with 3 × 50 mL of ethyl acetate. The organic phase was dried with MgSO4 and filtered. Using column chromatography, 50%, 361 mg of target product was obtained with hexane/ethylacetate (19:1). Mp: 91.5–94.2 °C; 1H NMR (500 MHz, CDCl3) δ 7.16 (d, J = 8.5 Hz, 2H), 6.95 (d, J = 2.2 Hz, 2H), 6.9 (d, J = 8.5 Hz, 2H), 6.28 (d, J = 2.2 Hz 2H), 3.7 (brs (NH), 2H). 13C NMR (125 MHz, CDCl3): δ 144.6, 132.9, 122.5, 119.8, 115.7, 109.5. LCMS calculated C10H10N2 + H, 159.092 [M + H]+, found 159.0983 [M + H]+.
Synthesis of 4-(4-(1H-pyrrol-1-yl)phenyl)-4H-dithieno[3,2-b:2',3'-d]pyrrole (DTP-Ph-Pyr)
The synthesized compound 1 was added to the 3,3'-dibromo-2,2'-bithiophene compound. it was stirred in toluene (10 mL) under argon gas. Pd2dba3 (25 mg, 0.0264), 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (BINAP), 40 mg, 0.0646 mmol and sodium tert-butoxide (NaOtBu), 153 mg, 1.5870 mmol was added, respectively. The mixture was stirred at 110 °C for 24 h. Then 20 mL of distilled water was added, and the water phase was washed with 3 × 50 mL of ethyl acetate. The organic phase was dried with MgSO4 and filtered. Using column chromatography, 21%, 20 mg target product was obtained with hexane. Mp: 189.4–192.6 °C; 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 8.8 Hz, 2H), 7.56 (d, J = 8.8 Hz, 2H), 7.23–7.17 (m, 4H), 7.15 (d, J = 2.2 Hz) 2H), 6.41 (d, J = 2.2 Hz 2H).13C NMR (125 MHz, CDCl3): δ 144.1, 138.8, 137.6, 124.0, 123.9, 121.9, 119.6, 117.2, 112.2, 111.0. LCMS calculated C18H13N2S2 + H, 321.052 [M + H]+, found 321.083 [M + H]+.
Results and discussion
Spectroscopic studies for monomer
Absorption and emission spectra of the compound DTP-Ph-Pyr (1 × 10–5 M) were achieved in ACN solvent medium as shown in Fig. 1. In this part, the optical behavior of monomer DTP-Ph-Pyr was compared with that of SNS-Ph-Pyr and other DTP derivatives. DTP-Ph-Pyr monomer gives a strong peak at about 305 nm with a shoulder peak at about 281 nm. At lower wavelengths, it gives a not very strong absorbance peak at 235 nm. Other DTP monomers in literature have also same behavior [40, 41]. In the preliminary studies, it was observed that the N-(4-(1H-pyrrol-1-yl)phenyl)-structure has two distinct absorbance peaks at 200 nm and 265 nm, which belong to pyrrole and phenyl units, respectively. In the case of SNS-Ph-Pyr, it gave very discrete peaks at three different wavelengths (200 nm, 265 nm, and 365 nm) in ACN [24]. While the absorption bands of the SNS monomer consist of three clearly visible peaks separated from each other, when the structure transitions to the fused form (as in the same structure), it has been observed that the absorption bands of DTP are also intertwined, showing that the structure is more rigid and planar. DTP-Ph-Pyr had an emission peak at about 350 nm when it was excited at 305 nm and the emission band of SNS-Ph-Pyr monomer shifted to higher wavelengths (425 nm) than that of DTP-Ph-Pyr [24].
Electrochemical, spectroelectrochemical and electrochromic studies
The electrochemical behavior of DTP-Ph-Pyr monomer was investigated by cyclic voltammetry (CV) in an electrolyte solution consisting of 0.1 M TBAPF6 dissolved in DCM. (Fig. 2a). As seen from the first cycle of resulting voltammogram, DTP-Ph-Pyr monomer had an irreversible broad anodic oxidation peak at 0.6 V and a sharp peak 1.30 V versus Ag/AgCl. The oxidation potential value of monomer is similar to its analogues [25,26,27,28,29,30,31,32,33]. The electrochemical polymerization of monomer was achieved in an electrolyte solution consisting of 0.1 M TBAPF6, respectively, dissolved in DCM via repetitive cycling. (Fig. 2a). Furthermore, N-phenyl pyrrole substituted trimeric SNS monomer (SNS-Ph-Pyr) had an oxidation potential 0.8 V which was higher than that of DTP-Ph-Pyr, indicating donor property of DTP-Ph-Pyr unit, as expected [24]. It was observed that the anodic and cathodic current values increased with each increasing peak in CV data, indicating that a polymeric film was formed on the working electrode surface. For electrochemical behavior analysis of polymeric film, DTP-Ph-Pyr monomer coated on Pt disc electrode (25 cycled) was cleaned with DCM solvent in order to remove monomeric and oligomeric species. Afterwards, P(DTP-Ph-Pyr) film coated on Pt disc was inserted in the CV cuvette with other electrodes in 0.1 M TBAPF6/ACN medium (Fig. 2b). Inspection of Fig. 2b shows that a clear reversible redox couple at 0.97 V (doped) and 0.37 V (de-doped) were observed with a scan rate of 100 mV s−1. When compared electrochemical response of polymer films with that of corresponding SNS polymer analogue [24], P(DTP-Ph-Pyr) shows an extra doping peak at about 0.3 V versus Ag/AgCl. This could indicate some shorter chain structures or segments for P(DTP-Ph-Pyr) polymer film. There are also other DTP-based polymers showing same behavior [25, 30].
The electrochemical behavior of polymer film obtained via repetitive cycles on Pt disc electrode, was scanned anodically in monomer free medium (an electrolyte solution of 0.1 M TBAPF6/ACN) for scan rate dependence experiments. The experimental result is given in Fig. 3a. Figure 3a shows the electropolymerized polymer behavior in different scan rates between 20 and 100 mV s–1 with an increment of 20 mV s–1. It is found that the current densities of both doping and dedoping processes ascend linearly with increasing scan rate, indicating that a polymer film on working electrode were well coated and the redox behavior is a nondiffusional process (Fig. 3b) [7, 13, 16].
The polymer films formed after 25 consecutive cycles in the potential range of − 0.5 to 1.2 V on ITO electrode was washed with DCM and then dried. Monomer DTP-Ph-Pyr was electropolymerized from α-H unit of thiophene compound. In order to prove this, FTIR spectra of both monomer and its polymer were compared with each other. The FTIR spectra of polymer film P(DTP-Ph-Pyr) and its corresponding monomer was recorded and given in supplementary data (Fig S1). The experimental studies showed that monomer had α-H and β-H of thiophene rings at 684 cm−1 and 830 cm−1, respectively. After, polymerization step, the peaks were broadened, and their intensities decreased. While some peaks of monomer remained same, the peak at 684 cm−1 disappeared, indicating that polymerization occurred through the α-coupling of thiophene ring. The peaks at 738 cm−1, 1006 cm−1 and 3104 cm−1 correspond to α-H of pyrrole ring, which indicates polymerization did not occur through coupling of subunit pyrrole [24, 42, 43].
Spectroelectrochemical studies were experimented for conjugated polymers in order to get information about their band gap, interband charge states of conductive polymers upon oxidation. For this purpose, a fresh polymer was again coated on the ITO electrode surface at a constant potential and then cleaned with monomer free solvent in order to remove oligomeric and monomeric species. The electro-optical behavior of polymer film on ITO electrode was experimented by monitoring the changes of the electronic absorption spectra under voltage pulses in 0.1 M TBAPF6/ACN (Fig. 4). The electronic absorption spectra of P(DTP-Ph-Pyr) polymer film exhibited an absorption band (\({\lambda }_{{\text{max}}}\)) at about 460 nm with a shoulder at 345 nm in its neutral state. The absorption band at 460 nm indicates π–π* transition of polymer film. Upon oxidation, these two bands (at 345 nm and 460 nm) lose intensity and accompanied new band at 680 nm. Upon further oxidation beyond 0.3 V, a new band around 950 nm also starts to intensify. Appearance of these two new bands shows the charge carrier formations of polymer. The changes in the electro-optical spectra also accompanied by color transitions in between orange and blue colors in the neutral and oxidized states, respectively. This behavior of polymer film indicates that P(DTP-Ph-Pyr) has an electrochromic property. The band gap (Egap) value of P(DTP-Ph-Pyr) coated on ITO electrode was also calculated from the commencement of low energy end of π–π* transition via electro-optical absorption spectra data. The experimental results showed that Egap of P(DTP-Ph-Pyr) was calculated as 2.03 eV.
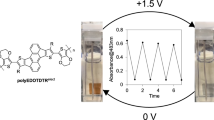
Electrochromic properties of conjugated polymers can be characterized with some important parameters such as coloration efficiency (CE), switching times (t) and optical contrast (ΔT%). For this purpose, P(DTP-Ph-Pyr) polymer film deposited on ITO electrode was investigated under square wave input of − 0.5 V and 1.2 V in 10 s intervals by monitoring kinetic responses of the film at 460 nm, 680 nm, and 950 nm and experimental results and data related electrochromic properties are summarized in Fig. 5 and Table 1, respectively. Considering the first switching cycles, ΔT% values were found as 26.5%, 26.5% and 52.5% for 480 nm, 680 nm, and 950 nm, respectively (Fig. 6a–c). The photographs of polymer film coated on ITO in the neutral and oxidized states are also shown in Fig. 6d. Since the color change requires some time to reach 95% of the maximum change upon oxidation and reduction of polymer film, the required time data colored to bleach (t95%b) or bleached to colored (t95%c) transitions are also summarized in Table 1.
CE is related with the power efficiency needed to change color during switching between its redox states and can be calculated by using the following equations [34].
where \({Q}_{d}\) is the charge density during charge transition step; \({T}_{{\text{colored}}}\) is transmittance in the oxidized states and \({T}_{{\text{bleached}}}\) is the transmittance in the neutral state. The CE of polymer film was calculated from Eqs. (1 and 2) and found as 80 cm2/C at 460 nm, 54 cm2/C at 680 nm and 123 cm2/C at 950 nm. Electrochromic properties of polymers can be specified with regard to CIE 1976 (L, a, b) color space with daylight (Standard Illuminant D65/10 as illuminant and 10°). L, a, and b are interrelated with the parameter of the lightness, red-green and yellow-blue balances, respectively. These values are added into Table 1 for color standardization purposes.
First of all, the effect of fused DTP unit on the electrochromic and optical properties of the polymer films, deposited on ITO electrodes was investigated. For that reason, these properties of both P(SNS-Ph-Pyr) and P(DTP-Ph-Pyr) are summarized in Table 1. For P(SNS-Ph-Pyr) polymer film, \({\lambda }_{{\text{max}}}\) was found as 383 nm in its neutral state [24], which is lower than that of P(DTP-Ph-Pyr) (460 nm). As a result, the band gap of P(DTP-Ph-Pyr) found lower than that of P(SNS-Ph-Pyr) and the neutral color shifted from yellow (color for P(SNS-Ph-Pyr) in the neutral state) to orange (color for P(DTP-Ph-Pyr) in the neutral state). This indicates that fusion of trimeric units for P(DTP-Ph-Pyr) creates outperformed conjugation, rigid planarity and shifts to longer wavelengths when compared with that of non-fused formation, SNS polymer analogue. Large angles in between consecutive trimeric SNS structure decreases the π-electron delocalization along the main chain backbone and increases the band gap. Table 1 shows that the CE values of P(SNS-Ph-Pyr) polymer were higher than those of P(DTP-Ph-Pyr). As seen in Eq. (1), although optical contrast of the P(DTP-Ph-Pyr) polymer were higher than that of the P(SNS) derivative, it can be said that the charge density value of the P(DTP-Ph-Pyr) was higher and more effective than that of the P(SNS-Ph-Pyr) derivative. This implies that main chain structure does play more dominant role during the determination of the band gap and electrochromic properties of conjugated polymers.
The influence of subunit for DTP structure on spectroelectrochemical and electrochromic properties of corresponding polymers were also studied and results were depicted in supplementary document (Table SI1). The band gap values of conjugated polymers depend on some properties of polymer such as, π conjugation, bond-length alternation, resonance energy, planarity, substituent effect, intermolecular interactions [44]. The band gap values of N-aryl substituted P(DTP) derivatives generally change from 1.65 to 2.07 eV, depending on the degree of intramolecular π-overlapping (electron delocalization) and torsional angles/steric effect of bulky units, which cause a change the length of polymer conjugation [25,26,27,28,29,30,31,32,33, 45] (see Table SI1). The band gap value of P(DTP-Ph-Pyr) (2.03 eV) was found to be slightly higher as compared to the previous analogues containing different side chains. These different data may also be related some factors such as polymer length, stacking of structure, polymer planarity, etc [46]. While the electrochromic parameters changed according to the functional groups attached to the P(DTP) polymer, it was found that the optical contrast values in the visible region were generally higher than those in the near-IR region. A similar result was also observed for the P(DTP-Ph-Pyr) polymer. When looking at the colors achieved, yellow, brown, and red tones were obtained in the neutral state, while blue and tones were obtained in the oxidized state. It can be said that switching times are generally low and generally close to 2 s. When these properties of P(DTP-Ph-Pyr) polymer were compared with for other DTP-based polymers in Table SI1, P(DTP-Ph-Pyr) polymer structure has highest ΔT% and CE values than others have [26, 28, 29, 32, 45]. Furthermore, it can be derived that all had higher ΔT% and CE values in the near infrared region than those in the visible region.
Conclusions
With this study, a phenyl-pyrrole subunit was attached to the central nitrogen atom of the DTP unit. Its corresponding polymer, P(DTP-Ph-Pyr), was electrochemically synthesized via potential cycling in the medium of 0.1 M TBAPF6/DCM. Electrochemical and spectroelectrochemical experiments showed that polymer film can reversibly be switched in between neutral and oxidized states. Electrochromic properties of P(DTP-Ph-Pyr) were studied in detail. Polymer gave a maximum absorption peak at 460 nm in its neutral state, which has an orange color and shades of blue upon applying positive voltages. The experimental results showed that electrochromic polymer had high optical contrast and coloration efficiency value in near infrared region of spectrum. This indicates that this polymer can be a promise candidate for near infrared region detectors and electrochromic device applications. The band gap of polymer film was calculated as 2.03 eV. The effect of fused structure and subunit on the electrochemical and optical properties of P(DTP-Ph-Pyr) was tried to be analyzed and these properties were compared with those of its DTP analogue polymers and its sister polymer, P(SNS-Ph-Pyr). As a result of these findings, it can be concluded that the type of fused monomer structure causes a distinctive effect on electrochemical and spectroelectrochemical properties for its corresponding polymer. For instance, the fusion of SNS structure in polymer structure lowered the band gap enhances optical contrast and switching times. Furthermore, among the DTP electrochromic homopolymers with aryl subunits, P(DTP-Ph-Pyr) has the highest optical contrast (26.5% at 460 nm, 26.5% at 680 nm, 52.5% at 950 nm) and coloration efficiency (80 cm2/C at 460 nm, 54 cm2/C at 680 nm, 123 cm2/C at 950 nm) values. We believe that the DTP monomer is a chemical platform that can be easily functionalized, just like the SNS monomer. Therefore, it is hoped that DTP homopolymers will become even more popular for electrochromic purposes in the future.
References
Yan Y, Jiang Y, Ng ELL, Zhang Y, Owh C, Wang F, Chan BQY (2023) Progress and opportunities in additive manufacturing of electrically conductive polymer composites. Mater Today Adv 17:100333
Kim J, Lee J, You J, Park M-S, Hossain MS, Al Yamauchi Y, Kim JH (2016) Conductive polymers for next-generation energy storage systems: recent progress and new functions. Mater Horizons 3:517–535
Shi Y, Peng L, Ding Y, Zhao Y, Yu G (2015) Nanostructured conductive polymers for advanced energy storage. Chem Soc Rev 44:6684–6696
Bezgin Carbas B, Gulen M, Tolu MC, Sonmezoglu S (2017) Hydrogen sulphate-based ionic liquid-assisted electro-polymerization of PEDOT catalyst material for high-efficiency photoelectrochemical solar cells. Sci Rep 7:11672
Yildiz HB, Cevik E, Bezgin Carbas B (2019) In: Thomas S, Grohens Y, Pottathara N (eds) Micro and nano technologies. Elsevier, Amsterdam, pp 65–89
Sirringhaus H, Tessler N, Friend RH (1998) Integrated optoelectronic devices based on conjugated polymers. Science 280:1741–1744
Bezgin Carbas B, Asil D, Friend RH, Önal AM (2014) Org Electron Phys Mater Appl 15:500–508
BezginCarbas B, Tekin B (2018) Poly(3,4-ethylenedioxythiophene) electrode grown in the presence of ionic liquid and its symmetrical electrochemical supercapacitor application. Polym Bull 75:1547–1562
Feng X, Liang Y, Zhi L, Thomas A, Wu D, Lieberwirth I, Kolb U, Müllen K (2009) Synthesis of microporous carbon nanofibers and nanotubes from conjugated polymer network and evaluation in electrochemical capacitor. Adv Func Mater 19:2125–2129
Ergun EGC, BezginCarbas B (2022) Electrochromic copolymers of 2,5-dithienyl-N-substituted-pyrrole (SNS) derivatives with EDOT: properties and electrochromic device applications. Mater Today Commun 32:103888
Neo WT, Ye Q, Chua S-J, Xu J (2016) Conjugated polymer-based electrochromics: materials, device fabrication and application prospects. J Mater Chem C 4:7364–7376
Lee K, Povlich LK, Kim J (2010) Recent advances in fluorescent and colorimetric conjugated polymer-based biosensors. Analyst 135:2179–2189
Bezgin Carbas B, Kivrak A, Zora M, Önal AM (2012) Synthesis and electropolymerization of a new ion sensitive ethylenedioxy-substituted terthiophene monomer bearing a quinoxaline moiety. J Electroanal Chem 677–680:9–14
Cevik E, Bezgin CB, Senel M, Yildiz HB (2018) Construction of conducting polymer/cytochrome C/thylakoid membrane based photo-bioelectrochemical fuel cells generating high photocurrent via photosynthesis. Biosens Bioelectron 113:25–31
Oyaizu K, Nishide H (2009) Radical polymers for organic electronic devices: a radical departure from conjugated polymers? Adv Mater 21:2339–2344
Beaujuge PM, Reynolds JR (2010) Color control in π-conjugated organic polymers for use in electrochromic devices. Chem Rev 110:268–320
Camurlu P (2014) Polypyrrole derivatives for electrochromic applications. RSC Adv 4:55832–55845
Bezgin Carbas B (2022) Fluorene based electrochromic conjugated polymers: a review. Polymer 254:125040
Mortimer RJ (1997) Electrochromic materials. Chem Soc Rev 26:147–156
Morin J-F, Leclerc M, Adès D, Siove A (2005) Polycarbazoles: 25 years of progress. Macromol Rapid Commun 26:761–778
Ferraris JP, Newton MD (1992) Electrochemical and optical properties of thiophene-alkylheteroaromatic copolymers. Polymer 33:391–397
Ferraris JP, Andrus RG, Hrncir DC (1989) Steric effects on the optical and electrochemical properties of N-substituted pyrrole–thiophene monomers and polymers. J Chem Soc Chem Commun 8:1318–1320
Bezgin Carbas B, Ergun EGC (2022) A classified and comparative review of poly (2, 5-dithienyl-N-substituted-pyrrole) derivatives for electrochromic applications. Eur Polym J 175:111363
Bezgin Carbas B, Ergin NM, Yildiz HB, Kivrak A, Demet AE (2023) Electrochromic properties of a polydithienylpyrrole derivative with N-phenyl pyrrole subunit. Mater Chem Phys 293:126916
Rasmussen SC, Evenson SJ (2013) Dithieno [3,2-b:2′,3′-d] pyrrole-based materials: synthesis and application to organic electronics. Progress Polym Sci 38:1773–1804
Udum YA, Yıldız HB, Azak H, Sahin E, Talaz O, Çırpan A, Toppare L (2014) Synthesis and spectroelectrochemistry of dithieno (3,2-b:2′,3′-d) pyrrole derivatives. J Appl Polym Sci 131:14
Berlin A, Pagani G, Zotti G, Schiavon G (1992) Electrochemical polymerization of 1H, 7H-pyrrolo [2′,3′:4,5]-thieno [3,2-b] pyrrole and 4H-dithieno [3,2-b;2′,3′-d] pyrrole. Die Makromolekulare Chemie 193:399–409
Ogawa K, Rasmussen SC (2006) N-Functionalized poly (dithieno [3,2-b:2′,3′-d] pyrrole)s: highly fluorescent materials with reduced band gaps. Macromolecules 39:1771–1778
Förtsch S, Bäuerle P (2017) Synthesis and characterization of two isomeric dithienopyrrole series and the corresponding electropolymers. Polym Chem 8:3586–3595
Azak H, Yildiz HB, Bezgin Carbas B (2018) Synthesis and characterization of a new poly (dithieno (3,2-b:2′,3′-d) pyrrole) derivative conjugated polymer: its electrochromic and biosensing applications. Polymer 134:44–52
Bandera Y, Jones HW, Grant B, Mell S, Foulger SH (2022) Synthesis, electropolymerization and functionalization via click chemistry of N-alkynylated dithieno [3,2-b:2′,3′-d] pyrrole. RSC Adv 12:29187–29196
Rybakiewicz R, Skorka L, Louarn G, Ganczarczyk R, Zagorska M, Pron A (2019) N-substituted dithienopyrroles as electrochemically active monomers: synthesis, electropolymerization and spectroelectrochemistry of the polymerization products. Electrochim Acta 295:472–483
Kivrak A, Yıldız HB, Gökyer S, Bezgin Carbas B (2018) Turk J Chem 42:439–447
Rybakiewicz-Sekita R, Toman P, Ganczarczyk R, Drapala J, Ledwon P, Banasiewicz M, Skorka L, Matyjasiak A, Zagorska M, Pron A (2022) DAD compounds combining dithienopyrrole donors and acceptors of increasing electron-withdrawing capability: synthesis, spectroscopy, electropolymerization, and electrochromism. J Phys Chem B 126:4089–4105
Wu TY, Li JL (2016) Electrochemical synthesis, optical, electrochemical and electrochromic characterizations of indene and 1,2,5-thiadiazole-based poly (2,5-dithienylpyrrole) derivatives. RSC Adv 6:15988–15998
Cevher D, Toppare L, Cirpan A (2023) Structure-electrochemical property relationship of f vs 2f substituents in low bandgap conjugated polymers. J Electrochem Soc 170:060524
Dikcal F, Ozturk T, Cınar ME (2017) Fused thiophenes: an overview of the computational investigations. Org Commun 10:56–71
Skabara PJ (2009) In: Perepichka IF, Perepichka DF (eds) Handbook of thiophene-based materials, vol 3. Wiley, Chichester
Wang Y, Wang J, Li GX, He G, Chen G (2017) Halogen-bond-promoted photoactivation of perfluoroalkyl iodides: a photochemical protocol for perfluoroalkylation reactions. Org Lett 19:1442–1445
Fujitsuka M, Sato T, Sezaki F, Tanaka K, Watanabe A, Ito O (1998) Laser flash photolysis study on the photoinduced reactions of 3,3′-bridged bithiophenes. J Chem Soc Faraday Trans 94:3331–3337
Ogawa K, Rasmussen SC (2003) A simple and efficient route to N-functionalized dithieno [3,2-b:2′,3′-d] pyrroles: fused-ring building blocks for new conjugated polymeric systems. J Org Chem 68:2921–2928
Just PE, Chane-Ching KI, Lacroix JC, Lacaze PC (2001) Electrochemical oxidation of dipyrrolyl derivatives: application to the formation of reticulated conducting polymers with conjugated spacers. Electrochim Acta 46:3279–3285
Cihaner A, Mert O, Demir AS (2009) A novel electrochromic and fluorescent polythienylpyrrole bearing 1,1′-bipyrrole. Electrochim Acta 54:1333–1338
Scharber MC, Sariciftci NS (2021) Low band gap conjugated semiconducting polymers. Adv Mater Technol 6:2000857
Hu B (2021) Neutral black color showing electrochromic copolymer based on dithienopyrroles and benzothiadiazole derivatives. ECS J Solid State Sci Technol 10:076003
Carbas BB, Yildiz HB (2024) A review of dithieno [3, 2-b: 2′, 3′-d] pyrrole-based electrochromic conjugated polymers. Eur Polym J 204:112700
Acknowledgements
We dedicate this article to our dear friend Nurseli Mislina Ergin, who contributed a lot to this study and unfortunately lost her life in the earthquake on 06.02.2023 in Kahramanmaraş, Türkiye. We thank the Scientific and Technological Research Council of Turkey (TUBITAK Grant Number 121Z142) for its financial support.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bezgin Carbas, B., Ergin, N.M., Yildiz, H.B. et al. Electrochemical and optical properties of poly(4-(4-(1H-pyrrol-1-yl)phenyl)-4H-dithieno[3,2-b:2',3'-d]pyrrole. Polym. Bull. 81, 9073–9089 (2024). https://doi.org/10.1007/s00289-024-05139-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-024-05139-7