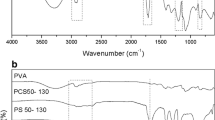
Abstract
The synthesis of polyacrylamide and a copolymer of acrylamide and maleic acid was studied depending on the pH, duration, temperature, and concentration of the redox system of the initiator. The process of alkaline hydrolysis of synthesized polymers has been studied. High-viscosity characteristics of polymer solutions were taken as the main criterion. To study the effect of pH, radical polymerization was carried out at pH values created by monomers in the system and in slightly alkaline and strongly alkaline media. It was found that with the participation of the initiating system, potassium persulfate and sodium sulfite in an amount of 0.1% of the mass of monomers in a 10% solution of acrylamide at a temperature of 40 °C and pH of about 2.2, after 3 h of polymerization, the highest yields of a high-molecular product are achieved. This product is characterized by polyacrylamide with a molecular weight of about 1000 kDa, and the proportion of oligomeric compounds in this mixture does not exceed 20%. To maintain higher yields of the copolymerization reaction of acrylamide and maleic acid, the process must be carried out for more than 4 h at a molar ratio of acrylamide and maleic acid of 7:1 and values of pH = 8.0–8.1. The resulting copolymer is characterized by molecular weight values of about 2500–2800 kDa. It was found that the hydrolysis of synthesized samples at a temperature of more than 80 °C results in an increase in molecular weight, which is associated with a decrease in the proportion of oligomeric compounds in the resulting mixture. Due to an increase in the average molecular weight and the transition of functional groups to carboxylate as a result of hydrolysis, their viscosity characteristics increase.













Similar content being viewed by others
References
Alnuaimi GH (2005) Synthesis and solution characterization of water-soluble polyacrylamide and its applications in oil industries. Theses, p 384. https://scholarworks.uaeu.ac.ae/all_theses/384
Sharipova AA, Aidarova SB, Mutaliyeva BZ, Babayev AA, Issakhov M, Issayeva AB, Madybekova GM, Grigoriev DO, Miller R (2017) The use of polymer and surfactants for the microencapsulation and emulsion stabilization. Colloids Interfaces 1(1):3. https://doi.org/10.3390/colloids1010003
Meyers RA (2002) Encyclopedia of physical science and technology. 3 rd ed., Academic Press
Kasgoz H, Özgümüş S, Orbay M (2003) Modified polyacrylamide hydrogels and their application in removal of heavy metal ions. Polymer 44(6):1785–1793. https://doi.org/10.1016/S0032-3861(03)00033-8
Kaşgöz H (2006) New sorbent hydrogels for removal of acidic dyes and metal ions from aqueous solutions. Polym Bull 56:517–528. https://doi.org/10.1007/s00289-006-0515-5
Mohsen A, Mohammed H (2012) A review: studies on uranium removal using different techniques overview. J Disper Sci Tech. https://doi.org/10.1080/01932691.2012.657954
Demirbas O, Turhan Y, Alkan M (2015) Thermodynamics and kinetics of adsorption of a cationic dye onto Sepiolite. Desalin Water Treat 54(3):707–714. https://doi.org/10.1080/19443994.2014.886299
Shkumat AP (2011) Search for new phosphors with specified physicochemical and chemical properties IX. New water-soluble copolymers of acrylamide. Vesnik of the Kharkiv. 20(43):74–83
Singh RP, Jain SK, Lang N (1991) Polymer science contemporary themes, S. Sivarm (Ed.), Vol II, p 716. Tata McGraw-Hill, New Delhi
Sojka RE, Bjorneberg DL, Entry JA, Lentz RD, Orts WJ (2007) Polyacrylamide in agriculture and environmental land management. Adv Agron 92:75–162
Chernyak MY et al (2018) Synthesis and study of hydrogen polymers of furaldehyde and levulinic acid. J SIBFU Chem 2(11):273–280. https://doi.org/10.17516/1998-2836-0074
Zhao T, Xing J, Dong Z, Tang Y, Wanfen P (2015) Synthesis of polyacrylamide with superb salt-thickening performance. Ind Eng Chem Res 54(43):10568–10574. https://doi.org/10.1021/acs.iecr.5b02718
Çolakoğlu GN, Çatıker E, Öztürk T, Meyvacı E (2022) Synthesis and characterization of brush-type polyβ-alanine-grafted polymethyl methacrylate using "grafting through method. Chem Pap 76(2):869–878. https://doi.org/10.1007/s11696-021-01908-0
Al-Sabagh AM, Kandile NG, El-Ghazawy RA et al (2013) Synthesis and characterization of high molecular weight hydrophobically modified polyacrylamide nanolatexes using novel nonionic polymerizable surfactants. Egypt J Pet 22(4):531–538. https://doi.org/10.1016/j.ejpe.2013.11.007
Pang X, Cheng G, Lu S, Tang E (2006) Synthesis of polyacrylamide gel beads with electrostatic functional groups for the molecular imprinting of bovine serum albumin. Anal Bioanal Chem 384(1):225–230. https://doi.org/10.1007/s00216-005-0147-x
Herth GS, Gunnar l, Buchholz F (2015) Polyacrylamides and poly (Acrylic Acids). Ullmann's encyclopedia of industrial chemistry. Weinheim Wiley VCH. 1–16. https://doi.org/10.1002/14356007.a21_143.pub2
Polyacrylamide (2013) Hazardous substances data bank. United States National Library of Medicine (2003). Consumption Patterns. CASRN: 9003–05–8. Retrieved
Siyam TE (2001) Development of acrylamide polymers for the treatment of waste water. Des Monomers Polym 4:107–168. https://doi.org/10.1163/156855500300203377
Ben-Hur M, Malik M, Letey J, Mingelgrin U (1992) Polyacrylamide in agriculture and environmental land management. Adv Agron 92:75–162
Levy GJ, Agassi M (1995) Polymer molecular weight and degree of drying effects on infiltration and erosion of three different soils. Aust J Soil Res 33:1007–1018
Mamedov AI, Beckmann S, Huang C, Levy GJ (2007) Aggregate stability as affected by polyacrylamide molecular weight, soil texture, and water quality. Soil Sci Soc Am J 71:1909–1918. https://doi.org/10.2136/sssaj2007.0096
Ernest F (1992) Silversmith Free-radical polymerization of acrylamide. J Chem Educ 69(9):763. https://doi.org/10.1021/ed069p763.1
Kuldasheva S, Jumabaev B, Agzamkhodjayev A, Aymirzaeva L, Shomurodov K (2015) Stabilization of the moving sands of the drained and dried aral sea bed. J Chem Tech Met 50(3):314–320
Riggs JP, Rodriguez F (1967) Persulfate-initiated polymerization of acrylamide. J Polym Sci A 1 Polym Chem 5:3151–3165. https://doi.org/10.1002/pol.1967.150051215
Tasdelen M, Karagoz B, Bicak N et al (2008) Phenacylpyridinium oxalate as a novel water-soluble Photoinitiator for free radical polymerization. Polym Bull 59:759–766. https://doi.org/10.1007/s00289-007-0822-5
Borai EH, Hamed MG, El-Kamash AM et al (2016) Synthesis, characterization and application of poly (acrylamide-maleic Acidacrylonitrile) by gamma irradiation induced grafting polymerization. Benha J Appl Sc 1(1):53–61. https://doi.org/10.21608/BJAS.2016.160300
Lipin AA, Shibashov AV, Lipin AG (2015) Kinetics of polymerization of acrylamide in concentrated aqueous solutions. J Appl Chem 88(1):103–108. https://doi.org/10.1134/S1070427215010140
Baimuratova RK, Dzhardimalieva GI et al (2021) Novel self-healing Metallocopolymers with pendent 4-phenyl-2, 20:60, 200-terpyridine ligand: kinetic studies and mechanical properties. Polymers 13:1760. https://doi.org/10.3390/polym13111760
Nedal Y, Abu-Thabit, (2017) Thermochemistry of acrylamide polymerization: an illustration of auto-acceleration and gel effect. World J Chem Educ 5(3):94–101. https://doi.org/10.12691/wjce-5-3-3
Kishore K, Santhanalakshmi KN (1981) Thermal polymerization of acrylamide by differential scanning calorimetry. J Pol Sci Polym Chem Edit 19(10):2367–2375
Canterino PJ (1965) Chemical reactions of polymers. Science 148:66. https://doi.org/10.1126/science.148.3666.66.a
Plate NA, Noah OV, Stroganov LB (1983) Some problems of the theory of polymer-analogous and intramolecular reactions of macromolecules. Rev Polym Sci U.S.S.R 11:2603–2632. https://doi.org/10.1016/0032-3950(83)90337-4
Kurenkov VF, Hartan HG, Lobanov FI (2001) Alkaline hydrolysis of polyacrylamide. Russ J Appl Chem 74:543–554. https://doi.org/10.1023/A:101278682677
Sharipova AI, Akhmadjonov IL, Abdikamalova AB et al (2021) Synthesis of new fixings of mobile sands. Alinteri J Agricul Sci 36(1):356–361. https://doi.org/10.47059/alinteri/V36I1/AJAS21053
Sharipova AI, Khamraev SS (2008) Investigation of the stabilizing properties of a polyelectrolyte obtained on the basis of alkaline hydrolysis of acrylamide with sodium maleate. Uzb khim J 5:17–20
Sharipova AI, Akbarov HI, Andriyko LS (2020) The stabilizing effect of polyelectrolytes on bentonite suspensions of Karakalpakstan Republic. Ukrainian conference with international participation chemistry, Physics technology of surface, Kiev Ukraine
Gunari AA, Gundiah S (1981) Kinetics of alkaline hydrolysis of polyacrylamide in solution by viscosimetric technique. Die Makromolekulare Chemie 182(1):1–8. https://doi.org/10.1002/macp.1981.021820101
Fundamentals of analytical chemistry / edited by Zolotov YA (2004) M: Higher School, 2, 503
Yan F, Zheng Ch, Zhai X, Zhao D (1998) Preparation and characterization of polyacrylamide in cationic microemulsion. J Appl Polym Sci 67:747–754
Zeynali ME and Rabbii A (2002) Alkaline hydrolysis of polyacrylamide and study on poly (acrylamide-co-sodium acrylate) Properties. Iran Polym J 11
Bashkatov TV, Zhigalin Y (1987) Technology of synthetic rubbers 2nd ed. L.: Chemistry, p 360
YaM A, Semenova LG, Shapovalov VD et al (2015) Obtaining a low-molecular copolymer of Maleic anhydride with Styrene in a homogeneous solvent. Int J Exper Educ 9:106–110
Wu YM, Wang CX, Xu J (2010) Aqueous dispersion polymerization of amphoteric polyacrylamide. J Appl Polym Sci 115:1131–1137
Sun YL, Hui Xiang Du, Wang H, Huang YH (2011) Synthesis of super-high molecular weight polyacrylamides and their flocculation properties. Ad Mater Res 396–398:1667–1671. https://doi.org/10.4028/www.scientific.net/AMR.396-398.1667
Ju N, Zeng W (2000) A study of synthesis and flocculant properties of cation-polyacrylamide. Chem Ind Guangzhou 28(1):65–68
Ma J, Zheng H, Tan M et al (2013) Synthesis, characterization, and occulation performance of anionic polyacrylamide P (AM-AA-AMPS). J App Pol Sci 129(4):1984–1991. https://doi.org/10.1002/app.38900
Cai H, Meng F, Li C, Zhao F (2015) Aqueous solution polymerization of acrylamide: a pilot-scale study. ICMMCCE 2015:2714–2718. https://doi.org/10.2991/icmmcce-15.2015.523
Hiemenz PC, and Lodge TP (2007) Polymer chemistry (2nd ed.) CRC Press. https://doi.org/10.1201/9781420018271
Tileubaev SO et al. (2022) Investigation of inhibitory characteristics of polymer drilling fluids // Universum: technical sciences: 5(98). URL: https://7universum.com/ru/tech/archive/item/13697
Kasterina TN and Kalinina LS (1963) Chemical methods of research of synthetic resins and plastic masses. M.: Goskhimizdat, 284
Kurenkov VF, Verizhnikova AS, Myagchenkov VA (1986) Features of inversion emulsion polymerization of acrylamide in the presence of Tuon and the initiating system K2S2O8-Na2S2O5. High Mol Compd XXVIII 3:488–492
ASTM D2857–22 Standard practice for dilute solution viscosity of polymers
Tsuneyuki S, Kazuhiko N, Shigeki M, Takayuki O (1979) Radical polymerization of maleic acid by potassium persulfate in the presence of Polyvinylpyrrolidone in water. J Macro Sci A 13(6):751–766. https://doi.org/10.1080/00222337908056686
Lipin AA, Shibashov AV, Lipin AG (2014) Modeling of the polymerization process of acrylamide in concentrated aqueous solutions. Chem chem Tech 57(12):85–87
Ivanov VA, Kamenshchikov AF, Gromov VF, Kaminsky VA, Bune E (1992) Mathematical modeling of the polymerization process of acrylamide in concentrated aqueous solutions. High Mol Compd 34(9):15–21
Chapiro A (1981) Auto-acceleration in free-radical polymerizations under precipitating conditions. Polym Sci Overv. https://doi.org/10.1021/bk-1981-0175.ch016
Chapiro A (1977) Radiation induced grafting. Radiat Phys Chem 9:55–67. https://doi.org/10.1016/0146-5724(77)90072-3
Guryeva LL, Tkachuk AI, Javadyan EA et al (2007) Investigation of the kinetics and mechanism of anionic polymerization of acrylamide monomers. High Mol Compd A 49(9):1635–1648
Strepikheev AA, Derevitskaya VA, Slonimsky GL (1966) Fundamentals of chemistry of high-molecular compounds. Second edition, M, Publishing House Chemistry, p 516
Workshop on chemistry and physics of polymers. M: Chemistry (1977), p 547
Abdollahi Z, Frounchi M, Dadbin S (2011) Synthesis, characterization and comparison of PAM, cationic PDMC and P(AM-co-DMC) based on solution polymerization. J Ind Eng Chem 17:580–586
Rc N, Guo L, Cy X (2008) Study on synthesis and flocculation property of cation-polyacrylamide. J Coal Sci Eng China 14:143–146. https://doi.org/10.1007/s12404-008-0029-x
Rozenberg BA, Bogdanova LM, Dzhavadyan EA, Komarov BA, Boiko GN, Gur’eva LL, Estrina GA (2003) Mechanism of anionic polymerization of acrylates and methacrylates containing mobile hydrogen. Polym Sci Ser A 45:1–9
Çatıker E, Hamzaçebi A (2022) A novel strategy for poly(β-alanine-b-lactone)s: sequentially HTP and AROP. Macromol Res 30:305–313. https://doi.org/10.1007/s13233-022-0034-8
Tileubaev SO, Kalilaev MU, Abdikamalova AB, Eshmetov ID (2021) The influence of stabilizers on the technological characteristics of clay drilling fluids. Univ Chem Biol 8(86):41–45
Rosenberg GA, Komarov BA, Boyko KN et al (2001) Transformations of acrylates under the action of active polymerization centers—oxides initiated by tertiary amines. High Mol Compd A 43(8):1299–1307
Tileubaev SO, Abdikamalova AB, Kalilaev MU, Eshmetov ID (2022) Synthesis of stabilizers of drilling fluids based on acrylamide and their stabilizing effect. International scientific—online conference on innovation in the modern education system. USA, pp 295–301
Asanov AA, Pogorelsky KV, Sharipova AI (1995) Development of colloidal-chemical bases for obtaining a new water-soluble polymer-soil structurizer. 2nd Scientific conference of young scientists and specialists Fertilizers-95. Tashkent, p 68
Kheradmand H, François J, Plazanet V (1988) Hydrolysis of polyacrylamide and acrylic acid-acrylamide copolymers at neutral pH and high temperature. Polymer 29(5):860–870. https://doi.org/10.1016/0032-3861(88)90145-0
Semchikov YD (2003) Vysokomolekulyarnye soedineniya [High- molecular compounds]. N. Novgorod: Nizhegorodskiy gosudar-stvennyy universitet im. N. 1. Lobachevskogo Publ.; M.: Akademiya Publ., p 368
Çatıker E, Meyvacı E, Atakay M, Salih B, Öztürk T (2019) Synthesis and characterization of amphiphilic triblock copolymers including β-alanine/α-methyl-β-alanine and ethylene glycol by click chemistry. Polym Bull 76:2113–2128. https://doi.org/10.1007/s00289-018-2561-1
Stahl GA, Schulz DN (1988) Water-soluble polymers for petroleum recovery. Springer New York NY. https://doi.org/10.1007/978-1-4757-1985-7
Yakimova LB, Krutko ET (2022) Relative activity of methacrylamide and 2-acrylamide-2-methylpropansulbphonate sodium in the reaction of radical copolymerization. Poly Mat Tech 8(2):25–29
Abdikamalova A, Kuldasheva Sh, Eshmetov I, Abdurakhimov D et al (2022) Polymers as soil structure–forming agents. Overv Ann For Res 65(1):2797–2810
Kulicke WM, Böse N (1982) [η]-M-relationship for polyacrylamide in aqueous 0.1 M Na2SO4 solution. Polym Bull 7:205–210. https://doi.org/10.1007/BF00255316
Yakimova LB, Kyivitskaya DV (2018) Determination of copolymerization constants of sodium methacrylate and 2-acrylamido-2-methylpropanesulfonate sodium. J Belarusian St Univ Chem 1:76–82
Osmanov T, Kozlova NV (1991) Polymerization of acrylamide in concentrated aqueous solutions in the presence of alkaline hydrolyzing agents. Plastics 8:9–11
Bolshakov AI, Kiryukhin DYu (2007) Spontaneous polymerization of acrylamide in a mixture with glycerin. High Mol Compd A 49(9):1621–1627
Sáez-Plaza P, Navas MJ, Wybraniec S, Michałowski T, Asuero AG (2013) An overview of the Kjeldahl method of nitrogen determination. Part II. Sample preparation, working scale, instrumental finish, and quality control. Crit Rev Anal Chem 43(4):224–272
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuldasheva, S., Abdikamalova, A., Eshmetov, I. et al. Investigation of changes in the viscosity properties of acrylamide (co)polymer and their hydrolyzed forms depending on the conditions of their preparation. Polym. Bull. 81, 4065–4091 (2024). https://doi.org/10.1007/s00289-023-04875-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04875-6