Abstract
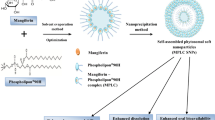
Galangin is a natural flavonol with high antioxidant properties and has a wide range characteristics of biological activity spectrum. Galangin has low solubility, permeability, and bioavailability, limiting its therapeutic use like many flavonoids. In this study, nano-sized polyelectrolyte liposomes were developed and characterized to overcome the properties that limit the use of galangin. The various parameters (phospholipid/solvent ratio, cholesterol/solvent ratio, time and galangin/solvent ratio) were optimized with a response surface methodology-central composite design to develop liposomes with maximum encapsulation efficiency using the thin-film hydration method. An optimum liposome formulation was developed with 93.77 ± 0.05% encapsulation efficiency, a spherical large unilamellar vesicle with a size of 485.5 ± 128.41 nm, and a zeta potential of − 48 ± 7 mV. The optimum liposome formulation was coated with polyelectrolyte biopolymer chitosan (CH) and gum arabic (GUA) using the layer-by-layer deposition method to improve its stability and drug release profile. The CH-liposome has a size of 208.05 ± 73.04 nm and a zeta potential of + 40 ± 5 mV. The GUA-CH-liposome has a size of 266.60 ± 8.49 nm and a zeta potential of − 6 mV. Fourier transform infrared spectroscopy analysis showed that galangin was encapsulated without disturbing the liposome structure and the polyelectrolyte coated the surface with electrostatic interaction. At the end of the in vitro release study, GUA-CH-liposome released 23.84% of galangin. Regarding stability, drug loading capacity of GUA-CH-liposome, which was 93.77 ± 0.05% on day 0, changed to 92.72 ± 0.51% at + 4 °C, 93.09 ± 0.01% at room temperature and 93.15 ± 0.01% at − 24 °C on day 28.





Similar content being viewed by others
References
Liang X, Wang P, Yang C et al (2021) Galangin inhibits gastric cancer growth through enhancing STAT3 mediated ROS production. Front Pharmacol 12:646628. https://doi.org/10.3389/fphar.2021.646628
Fang D, Xiong Z, Xu J et al (2019) Chemopreventive mechanisms of galangin against hepatocellular carcinoma: a review. Biomed Pharmacother 109:2054–2206. https://doi.org/10.1016/j.biopha.2018.09.154
Abubakar IB, Malami I, Yahaya Y, Sule SM (2018) A review on the ethnomedicinal uses, phytochemistry and pharmacology of Alpinia officinarum Hance. J Ethnopharmacol 224:45–62. https://doi.org/10.1016/j.jep.2018.05.027
Jeong D, Kim HK, Jeong JP et al (2016) Cyclosophoraose/cellulose hydrogels as an efficient delivery system for galangin, a hydrophobic antibacterial drug. Cellulose 23:2609–2625. https://doi.org/10.1007/s10570-016-0975-1
Subramani T, Ganapathyswamy H (2020) An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J Food Sci Technol 57:3545–3555. https://doi.org/10.1007/s13197-020-04360-2
Witika BA, Makoni PA, Matafwali SK et al (2021) Enhancement of biological and pharmacological properties of an encapsulated polyphenol: curcumin. Molecules 26:4244. https://doi.org/10.3390/molecules26144244
Saifullah M, Shishir MRI, Ferdowsi R et al (2019) Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: a critical review. Trends Food Sci Technol 86:230–251. https://doi.org/10.1016/j.tifs.2019.02.030
Shishir MRI, Xie L, Sun C et al (2018) Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci Technol 78:34–60. https://doi.org/10.1016/j.tifs.2018.05.018
Witika BA, Makoni PA, Matafwali SK et al (2020) Biocompatibility of biomaterials for nanoencapsulation: current approaches. Nanomaterials 10:1–40. https://doi.org/10.3390/nano10091649
Liu G, Hou S, Tong P, Li J (2022) Liposomes: preparation, characteristics, and application strategies in analytical chemistry. Crit Rev Anal Chem 52:392–412. https://doi.org/10.1080/10408347.2020.1805293
Nguyen TA, Tang QD, Doan DCT, Dang MC (2016) Micro and nano liposome vesicles containing curcumin for a drug delivery system. Adv Nat Sci Nanosci and Nanotechnol 7:035003. https://doi.org/10.1088/2043-6262/7/3/035003
Balakrishnan KRRP, Gopi S (2022) Systematic review on activity of liposomal encapsulated antioxidant, antibiotics, and antiviral agents. J Liposome Res 8:1–14. https://doi.org/10.1080/08982104.2021.2024568
Akhavan S, Assadpour E, Katouzian I, Jafari SM (2018) Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci Technol 74:132–146. https://doi.org/10.1016/j.tifs.2018.02.001
Su C, Liu Y, He Y, Gu J (2018) Analytical methods for investigating in vivo fate of nanoliposomes: a review. J Pharm Anal 8:219–225. https://doi.org/10.1016/j.jpha.2018.07.002
Apolinário AC, Hauschke L, Nunes JR, Lopes LB (2021) Lipid nanovesicles for biomedical applications: ‘what is in a name’? Prog Lipid Res 82:101096. https://doi.org/10.1016/j.plipres.2021.101096
Aguilar-Pérez KM, Avilés-Castrillo JI, Medina DI et al (2020) Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front Bioeng Biotechnol 8:579536. https://doi.org/10.3389/fbioe.2020.579536
Trucillo P, Campardelli R, Reverchon E (2020) Liposomes: From bangham to supercritical fluids. Processes 8:1022. https://doi.org/10.3390/pr8091022
Enaru B, Socaci S, Farcas A et al (2021) Novel delivery systems of polyphenols and their potential health benefits. Pharmaceuticals 14:946. https://doi.org/10.3390/ph14100946
Tan C, Wang J, Sun B (2021) Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol Adv 48:107727. https://doi.org/10.1016/j.biotechadv.2021.107727
Li J, Zhai J, Dyett B et al (2021) Effect of gum arabic or sodium alginate incorporation on the physicochemical and curcumin retention properties of liposomes. LWT 139:110571. https://doi.org/10.1016/j.lwt.2020.110571
Ahmed KS, Hussein SA, Ali AH et al (2019) Liposome: composition, characterisation, preparation, and recent innovation in clinical applications. J Drug Target 27:742–761. https://doi.org/10.1080/1061186x.2018.1527337
Kumar S, Dutta J, Dutta PK, Koh J (2020) A systematic study on chitosan-liposome based systems for biomedical applications. Int J Biol Macromol 160:470–481. https://doi.org/10.1016/j.ijbiomac.2020.05.192
Sabry S, El Hakim Ramadan A, Abd elghany M et al (2021) Formulation, characterization, and evaluation of the anti-tumor activity of nanosized galangin loaded niosomes on chemically induced hepatocellular carcinoma in rats. J Drug Deliv Sci Technol 61:102163. https://doi.org/10.1016/j.jddst.2020.102163
Patil S, Ujalambkar V, Rathore A et al (2019) Galangin loaded galactosylated pluronic F68 polymeric micelles for liver targeting. Biomed Pharmacother 112:108691. https://doi.org/10.1016/j.biopha.2019.108691
Hajipour H, Nouri M, Ghorbani M et al (2021) Targeted nanostructured lipid carrier containing galangin as a promising adjuvant for improving anticancer effects of chemotherapeutic agents. Naunyn-Schmiedeberg’s Arch Pharmacol 394:2353–2362. https://doi.org/10.21203/rs.3.rs-600166/v1
Zhu J, Wang Q, Li H et al (2018) Galangin-loaded, liver targeting liposomes: Optimization and hepatoprotective efficacy. J Drug Deliv Sci Technol 46:339–347. https://doi.org/10.1016/j.jddst.2018.05.034
Li Y, Guo M, Lin Z et al (2018) Multifunctional selenium nanoparticles with Galangin-induced HepG2 cell apoptosis through p38 and AKT signalling pathway. R Soc Open Sci 5:180509. https://doi.org/10.1098/rsos.180509
Yao H, Lu H, Zhang J et al (2019) Preparation of prolonged-circulating galangin-loaded liposomes and evaluation of antitumor efficacy in vitro and pharmacokinetics in vivo. J Nanomater. https://doi.org/10.1155/2019/7236895
Li CY, Cheng SE, Wang SH et al (2021) The anti-inflammatory effects of the bioactive compounds isolated from Alpinia officinarum Hance mediated by the suppression of NF-kappaB and MAPK signaling. Chin J Physiol 64:32–42. https://doi.org/10.4103/CJP.CJP_81_20
Karkar B, Şahin S (2022) Determination of phenolic compounds profiles and antioxidant properties of oleaster (Elaeagnus angustifolia L.) grown in Turkey. Eur Food Res Technol 248:219–241. https://doi.org/10.1007/s00217-021-03875-y
Şahin S, Nasir NTBM, Erken I et al (2019) Antioxidant composite films with chitosan and carotenoid extract from Chlorella vulgaris: Optimization of ultrasonic-assisted extraction of carotenoids and surface characterization of chitosan films. Mater Res Express 6:095404. https://doi.org/10.1088/2053-1591/ab2def
Cui T, Jia A, Yao M et al (2021) Characterization and caco-2 cell transport assay of chito-oligosaccharides nano-liposomes based on layer-by-layer coated. Molecules 26:4144. https://doi.org/10.3390/molecules26144144
Men W, Zhu P, Dong S et al (2020) Layer-by-layer pH-sensitive nanoparticles for drug delivery and controlled release with improved therapeutic efficacy in vivo. Drug Deliv 27:180–190. https://doi.org/10.1080/10717544.2019.1709922
Xiong Y, Guo D, Wang L et al (2009) Development of nobiliside A loaded liposomal formulation using response surface methodology. Int J Pharm 371:197–203. https://doi.org/10.1016/j.ijpharm.2008.12.031
Hashemi SH, Montazer M, Naghdi N, Toliyat T (2018) Formulation and characterization of alprazolam-loaded nanoliposomes: screening of process variables and optimizing characteristics using RSM. Drug Dev Ind Pharm 44:296–305. https://doi.org/10.1080/03639045.2017.1391834
Refaat H, Mady FM, Sarhan HA et al (2021) Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int J Pharm 592:120028. https://doi.org/10.1016/j.ijpharm.2020.120028
Deniz A, Sade A, Severcan F et al (2010) Celecoxib-loaded liposomes: Effect of cholesterol on encapsulation and in vitro release characteristics. Biosci Rep 30:365–373. https://doi.org/10.1042/BSR20090104
Maja L, Željko K, Mateja P (2020) Sustainable technologies for liposome preparation. J Supercrit Fluids 165:104984. https://doi.org/10.1016/j.supflu.2020.104984
Rasmussen MK, Pedersen JN, Marie R (2020) Size and surface charge characterization of nanoparticles with a salt gradient. Nat Commun 11:2337. https://doi.org/10.1038/s41467-020-15889-3
Wang DY, van der Mei HC, Ren Y et al (2020) Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front Chem 7:872. https://doi.org/10.3389/fchem.2019.00872
Barbosa JAC, Abdelsadig MSE, Conway BR, Merchant HA (2019) Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa. Int J Pharm X 1:100024. https://doi.org/10.1016/j.ijpx.2019.100024
Wang Y (2021) Liposome as a delivery system for the treatment of biofilm-mediated infections. J Appl Microbiol 131:2626–2639. https://doi.org/10.1111/jam.15053
Danaei M, Dehghankhold M, Ataei S et al (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10:57. https://doi.org/10.3390/pharmaceutics10020057
Wen MM, Farid RM, Kassem AA (2014) Nano-proniosomes enhancing the transdermal delivery of mefenamic acid. J Liposome Res 24:280–289. https://doi.org/10.3109/08982104.2014.911313
Perez-Ruiz AG, Ganem A, Olivares-Corichi IM, García-Sánchez JR (2018) Lecithin-chitosan-TPGS nanoparticles as nanocarriers of (-)-epicatechin enhanced its anticancer activity in breast cancer cells. RSC Adv 8:34773–34782. https://doi.org/10.1039/c8ra06327c
Tavares L, Noreña CPZ (2020) Encapsulation of ginger essential oil using complex coacervation method: coacervate formation, rheological property, and physicochemical characterization. Food Bioproc Tech 13:1405–1420. https://doi.org/10.1007/s11947-020-02480-3
Hernández-Fernández MÁ, García-Pinilla S, Ocampo-Salinas OI et al (2020) Microencapsulation of vanilla oleoresin (V. Planifolia andrews) by complex coacervation and spray drying: Physicochemical and microstructural characterization. Foods 9:1375. https://doi.org/10.3390/foods9101375
Rajabi H, Jafari SM, Rajabzadeh G et al (2019) Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids Surf A Physicochem Eng Asp 578:123644. https://doi.org/10.1016/j.colsurfa.2019.123644
Zhang T, Su M, Jiang X et al (2019) Transepithelial transport route and liposome encapsulation of milk-derived ACE-inhibitory peptide Arg-Leu-Ser-Phe-Asn-Pro. J Agric Food Chem 67:5544–5551. https://doi.org/10.1021/acs.jafc.9b00397
Jeon S, Yoo CY, Park SN (2015) Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surf B Biointerfaces 129:7–14. https://doi.org/10.1016/j.colsurfb.2015.03.018
Li N, Zhuang C, Wang M et al (2009) Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm 379:131–138. https://doi.org/10.1016/j.ijpharm.2009.06.020
Hermal F, Frisch B, Specht A et al (2020) Development and characterization of layer-by-layer coated liposomes to increase their resistance in biological media. Int J Pharm 586:119568. https://doi.org/10.1016/j.ijpharm.2020.119568
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karkar, B., Patır, İ. & Şahin, S. Development of galangin-loaded nano-sized polyelectrolyte liposome: optimization and characterization. Polym. Bull. 81, 2847–2867 (2024). https://doi.org/10.1007/s00289-023-04826-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04826-1