Abstract
Biocompatible polymers are attractive material for the manufacturing of surgical implants which break down in vivo without the necessity for a consequent operation for removal. Elaboration of composite biomaterials scaffolds as artificial bone graft materials remains a major task in bioengineering. Flaxseed mucilage was used as bioactive polysaccharide for preparing composite scaffolds made of calcium phosphate embedded in mucilage matrix. Calcium chloride was mixed with mucilage followed by the addition of phosphate precursor to stimulate the in situ formation of calcium phosphate. The obtained scaffolds mucilage/calcium phosphate at different pHs (5 and 8) were characterized using FTIR, XRD, TGA, SEM/EDX and TEM. The results showed the formation of two phases: mucilage/dicalcium phosphate dihydrate (MU/brushite) and mucilage/hydroxyapatite (MU/HA). MTT test was applied to evaluate viability of MC3T3-E1 osteoblasts cells, and the formed hybrids at various pH conditions were classified as non-cytotoxic. These findings establish the potential of developed composite to be used as bone graft substitute materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A bone fracture resulting in non-unions and defects due to trauma or degenerative conditions has become a prominent source of concern in orthopedic care. For bone repair, autografts and allografts remain the most popular options [1]. The clinical execution of these techniques is limited by donor site morbidity associated with autografting, disease transmission risk and known incidences of immunological rejection related to allografting [2]. Tissue engineering has advanced superior options in biobased functional substitutes and implants for the repair of damaged locations in response to this growing problem [3,4,5]. It entails delivering different drugs, bioactive molecules and precursor cells to the injured location using a scaffolding framework that should mimic the chemical and structural features of natural tissue [6]. An ideal tissue engineering matrix should provide optimal microenvironments to guide and regulate cellular attachment, migration, proliferation and differentiation [7, 8]
Bone is considered an amazing nano-composite performing critical functions for human physiology such as movement, production of red blood cells, protection to sensitive organs of the body such as brain and eye and storage to important minerals [9]. Bone is mainly composed of two phases: inorganic phase and organic phase. The inorganic phase (65%) is mainly made of calcium phosphate nanocrystals, especially hydroxyapatite (HAp, Ca10 (PO4)6(OH)2), while the organic phase (40%) is composed of collagen I fibrils [1]. For bone tissue engineering, calcium phosphate scaffolds have been extensively studied over the last few decades [10, 11]. Calcium phosphate-based bioceramics can generate a physiological response like that of bone, but their brittleness, limited mechanical strength and slow degradation rate prevent their applications. Polymers with higher bioactivity and mechanical strength exhibit a promising applications for diverse bone grafts [12, 13].
In recent years, composite materials that combine the benefits of both polymers and ceramics with significantly better mechanical characteristics and cell matrix interactions have carved a place in bone tissue engineering applications [14, 15]. The use of biopolymers with inorganic calcium phosphate has gotten a lot of interest in bone tissue engineering as pure polymeric systems. For example, cellulose nanofibers grafted with soy protein hydrolysate was prepared via amidation reaction. The bioactivity of the modified cellulose nanofibers was investigated via calcium phosphate mineralization in simulated body fluid, and the results showed the deposition of crystalline calcium phosphate resembling the natural apatite crystals of bone tissue. The cytotoxicity results established the high biocompatibility of the modified cellulose nanofibers, ascribable to the combined effect of bioactive protein and morphological changes of the nanofibrillar network [11]. These findings proposed a promising use of the composites of cellulose/calcium phosphate in the repair and/or the regeneration of hard tissues such as bone. Moreover, fabrication and designing of chitosan with inorganic calcium phosphates for promising applications in the field of bone regeneration, drug delivery, dental caries and wound dressing were reviewed in several new approaches [10].
Flaxseed holds about 3.0–9.0% mucilage which consists of polysaccharide chains of galacturonic acid (21.0–36.0%), xylose (19.0–38.0%), rhamnose (11.0–16.0%), galactose (12.0–16.0%), arabinose (8.0–13.0%) and glucose (4.0–6.0%) [16]. This work focused on an original usage of flax seed mucilage as a bioactive material for the calcium phosphate mineralization. It is proposed that this technology will increase to the available options for sustainable hybrid materials for bone tissue engineering. The bioactivity of the seed mucilage is achieved by hydrated polysaccharides (e.g., pectins, hemicelluloses and cellulose) [17]. Flax, plantain and cress species are recognized as typical seeds that can yield mucilage by hydration [18]. Several studies have been done to mimic properties of mucilage layers with polysaccharides or polymers for biomedical applications such as artificial joints [19] and hips [20]. The presence of polysaccharide layer on seed surfaces is a source of active functionalized carbohydrates for inorganic mineralization. The points of interest of polysaccharides are their availability, usually even biodegradability, their great biocompatibility, which they supply cell attachment and augmentation. However, because of their natural origin, they may cause immune responses. Flaxseed mucilage (Linum usitatissimum) is gained from soaking seeds in hot water. A pectic type of polysaccharide with a rhamnogalacturonan backbone forms the acidic fraction. The neutral polysaccharide in the mucilage is found in the form of β-D-xylan with Ara and Gal side chains [21].
The authors did not find any previous work that tried to use flaxseed mucilage as a biopolymer in bone tissue engineering. Therefore, a trial was conducted in this study to use a polysaccharide (flaxseed mucilage) with inorganic calcium phosphate in bone regenerative medicine. In the current article, flaxseed mucilage was extracted from flax seeds and applied to act as bioactive polysaccharide. The calcium and phosphate solutions were added as precursors to the flaxseed mucilage to form the calcium phosphate (at different pHs) for preparing cross-linked flaxseed mucilage/calcium phosphate composite which was further characterized with different techniques. The prepared composites at pH 5 and 8 were systematically investigated. To evaluate the bioactivity in the proliferation of connective tissue, the flaxseed mucilage/calcium phosphate hybrid materials prepared in the present work were tested with cell lines MC3T3-E1 osteoblasts cells.
Materials and methods
Flaxseed mucilage extraction
The mucilage extraction was carried out in accordance with our previously reported method [22]. First, flaxseed was bought from a local factory “Peacock” Tanta, Egypt. The seeds were hydraulic pressed and then subjected to defatting using a Soxhlet apparatus using n-hexane as defatting solvent. The defatted seals were spread to dry and then ground in coffee mill to obtain a finely divided material suitable for extraction studies. Second, mucilage was extracted by hot water: 200 g of seeds was stirred well by electric stirrer in (8 Liter) distilled water and then soaked for one h at 90 °C. The extract was centrifuged for 30 min at 3000 rpm and then passed through muslin cloth. The supernatant collected and concentrated by rotary evaporator at 80 °C. The polysaccharide was precipitated with the addition of ethanol to the final extract and then freeze-dried (Crest Alpha 1-4 LSC plus Germany). The powdered mucilage was crushed and kept in refrigerator until use.
Mucilage/calcium phosphate composites formation
Prior to the mineralization reaction, the starting solutions were prepared by dissolving the respective amounts (3.24 g) of dipotassium hydrogen phosphate K2HPO4 and calcium chloride CaCl2 (2.22 g) separately in 100 mL of water. Subsequently, flaxseed mucilage (0.6 g) was dissolved in the calcium chloride solution, and then, dipotassium hydrogen phosphate was added drop by drop. Adjust the solutions at both (5 and 8 pH) at 37 °C by magnetic stirrer and then left overnight. Samples were isolated by centrifugation at 3000 rpm for 30 min, and the white or off-white residues were freeze-dried (Crest Alpha 1-4 LSC plus Germany).
Characterization
Fourier transformed infrared spectra (FTIR) were obtained using a PerkinElmer FTIR spectrometer (PerkinElmer, USA) using KBr disks in the range of 4000–500 cm−1 with a resolution of 4 cm−1 and an accumulation of 16 scans per analysis. X-ray diffraction (XRD) patterns were recorded with an Empyrean Powder Diffractometer (Cu Kα, 0.154 nm) between 5 and 70° 2θ with a step size of 0.01°/sec. The surface morphology of the prepared mineralized scaffolds at different pHs was obtained using a scanning electron microscope (FEI-Quanta 2000, FESEM) attached with EDX Unit (energy-dispersive X-ray analyses). Transmission electron microscope (TEM) images were taken with a JEOL JEM-2100 electron microscopy at 100 k × magnification, with an acceleration voltage of 120 kV. Thermo-gravimetric analysis was performed on the PerkinElmer TGA thermo-gravimetric analyzer under air from 25 to 800 °C with a heating rate of 10°/min.
In vitro cell culture using MC3T3-E1 pre-osteoblastic cell
The pre-osteoblastic cells MC3T3-E1 (ATCC-CRL-2593; #99,072,810; Sigma) were maintained at 37 °C in a humidified atmosphere of 5% CO2, in ascorbate-free Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, USA) base media supplemented with 10% fetal calf serum (FCS), 1% antibiotic–antimycotic solution and 1 mM sodium pyruvate. Media was changed each two days, and cells were passaged using a 0.25% trypsin/EDTA solution at less than 80% confluence to maintain proliferation.
The cytotoxicity assay
The cytotoxicity assay was performed using MTT (3-4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide) assay in 96-well plates (104 cells/well) [21]. Typically, a suspension of MC3T3-E1 osteoblasts was cultured at different concentrations of neat flaxseed mucilage (MU) and MU hybrid samples for different time intervals (1, 3 and 5 d). The medium was changed every 3d. For MTT assay, 100 μL of the cell medium was removed and aliquots of 20 μl of MTT reagent solution (5 mg/ml) were added to the cell culture wells. After 4 h in dark, 100 μl of DMSO was added for solubilization to the culture plates to solubilize the formazan crystals, which formed via the reduction of MTT in living cells. Subsequently, the mean absorbance of each well was measured using ELISA reader at 570 nm. Three parallel experiments were performed.
Cell morphology
When cells reached 70% confluence, the different types of nanoparticles powder were added to the cells at a concentration of 10 μg/ml. After 48 h incubation, cell morphology was assessed using phase-contrast light microscope.
Statistical analysis
All experiments were done in triplicate, and the results were presented as mean ± standard deviation. Statistical analyses were performed with the one-way ANOVA test, by using Sigma Stat 3.5 software (Dundas Software Ltd, Toronto, Canada). P values ≤ 0.05 were considered statistically significant.
Results and discussion
Structure and morphological analysis
Flaxseed mucilage was extracted by the co-precipitated method which used ethanol to collect and precipitate mucilage. It has the potential for stabilizing emulsions, thickening foods and gelling solutions. It appears white in color due to freeze-drying methods applied for drying the watery mucilage after precipitation by ethanol. It has good functional properties like water- and oil-holding capacity. The dried mucilage is soluble in hot water and practically insoluble (precipitated) in an organic solvent. The chemical composition refers that it has appreciable amount of protein and ash. The chemical composition, water-holding capacity and oil-holding capacity of the flaxseed mucilage have been reported in our previous work, as shown in Table 1 [22].
FTIR analysis was investigated to illustrate the functional groups of the samples made with flaxseed mucilage and to clarify the interactions between mucilage and calcium phosphate. Figure 1 demonstrates the FTIR spectra of flaxseed mucilage and its corresponding mucilage/calcium phosphate composites. Flaxseed mucilage powder showed characteristic peaks at 2929 cm−1, related to the C–H bonds of the CH2–CH3 groups, at 1628 cm−1 to the vibration of the amide I, at 1400 cm−1 to C–OH uronic acid and at 1027 cm−1 to the C–O–C or C–OH bonds of pyranose [23,24,25]. Moreover, a broad absorbance of the hydroxyl (O–H) stretch in the region of approximately 3300 cm−1 was observed. This peak was significantly reduced after calcium phosphate formation and presented different shapes in the two composite samples which may reflect the precipitation of different calcium phosphate phases as shown in Fig. 1B and C. The formed flaxseed mucilage/calcium phosphate composites exhibit the absorption peaks corresponding to phosphate groups which appeared at 1110 cm−1 (P–O−3 mode), 520 cm−1 (P–O−4 mode) and 875 cm−1 (P–O−1 mode). The appearance of these peaks in the hybrid confirms the formation of calcium phosphate phases. Morover, the peak at 1628 cm−1 that corresponds to amide I vibration was decreased in intensity and shifted to higher wavenumber (1640 cm−1) in flaxseed mucilage/calcium phosphate composite. The changes in this peak reflect a type of interaction between flaxseed mucilage and calcium phosphate in the composite.
The XRD patterns for flaxseed mucilage and flaxseed mucilage/calcium phosphate composites formed at different pHs are displayed in Fig. 2. Pattern of flaxseed mucilage displays broad reflections at 9.8° and 20° 2θ, which can be attributed to the polysaccharide’s chains. This reflects the lack of crystallinity in the formed flaxseed mucilage sample. The XRD patterns of calcium phosphate precipitates as a function of the pH are also shown in Fig. 2 (B and C). It could be observed that calcium phosphates precipitated at pH 5 show different peaks at 2θ angles of 11.6, 20.9, 28.32, 29.3, 30.5, 34.2, 37.2, 42.1 and 50.16° which are related to brushite phase (CaHPO4·2H2O: ICDD file 00-009-0077) crystal formation. The sample prepared at pH 8 shows intense peaks at 2θ (°) = 25.9, 28.4, 31.9 and 38.9 which can be assigned to calcium phosphate hydroxyapatite phase (Ca5(PO4)3OH: ICDD file 00-024-0033). Our results are in complete agreement with Ferreira et al. [26] and Mekmene et al. [27], and the formation of brushite is promoted in acidic environments and that of HA at neutral or basic environments.
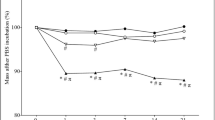
TGA studies were done to examine the thermal stability of flaxseed mucilage/calcium phosphate composites at pH 5 and pH 8. The TGA thermograms for flaxseed mucilage and flaxseed mucilage/calcium phosphate composites display three regions of weight loss, as shown in Fig. 3. The first stage of weight loss of flaxseed mucilage (A) (about 10%) detected below 60 °C, for flaxseed mucilage/calcium phosphate composites at pH 5 (B), this stage occurred below 93 °C (about 12%) and for flaxseed mucilage/calcium phosphate composites at pH 8 (C), this stage happened below 70 °C (about 4%). The first stage of weight loss in all samples could be recognized as water evaporation. The second stage of weight loss was detected between 60 and 226 °C, 93 and 195 °C and 70 and 240 °C for flaxseed mucilage (A), hybrid prepared at pH 5 (B) and that prepared at pH 8 (C), respectively. The weight loss in this stage was recorded around 17%, 20% and 11% for A, B and C samples, respectively. This stage shows the decomposition of organic parts present in the samples. After these two stages, the degradation of flaxseed mucilage (A) becomes fast until at 450 °C while the two composites decelerated. The total weight loss of flaxseed mucilage is about 82%, while for composites prepared at pH 5 it is about 43%, and for that prepared at pH 8, the total weight loss reaches about 23%. Overall, the TGA results reveal that the total weight loss for flaxseed mucilage > hybrid prepared at pH 5 > hybrid prepared at pH 8. This may be due to the entrance of flaxseed mucilage into the crystals of the hybrid prepared at pH 8 decreasing its weight loss.
The SEM images of the flaxseed mucilage/calcium phosphate composites prepared via calcium phosphate precipitation at two different pH are shown in Fig. 4. The formation of platelike structures was observed for the hybrid of flaxseed mucilage/calcium phosphate calcium phosphate at pH 5, as shown in Fig. 4A. However, precipitated calcium phosphate grew into agglomerated rods at pH 8 (Fig. 4B). According to the calcium/phosphate ratio at pH 8, the formation of hydroxyapatite should be stoichiometric (1.76), which indicates the formation of the hydroxyapatite crystal phase.
To visualize the internal structure of calcium phosphate formed using biomimetic processes, transmission electron microscopy (TEM) was used. As illustrated in Fig. 5, at pH 5, the formed calcium phosphate looks like plate-like structure with a diameter of 200 nm which approved the SEM morphology. This structure confirmed the XRD results which suggested the formation of dicalcium phosphate dihydrate at acidic solution. It was observed that at pH = 8, the mineralized calcium phosphate exhibited as rod-shaped nanocrystals. These crystals are near the hydroxyapatite internal structure. The rodlike shapes had a narrow size distribution within the nanometer range. In addition, the selected area electron diffraction (SAED) images reveal a diffraction pattern indicating poor crystalline structure of prepared hybrid materials. These results demonstrate that flaxseed mucilage can act as a biomimetic calcium phosphate mineralizing agent.
In vitro cytotoxicity studies of MU-based hybrids
The cell viability was determined by MTT assay. The result data of the cytotoxicity of neat flaxseed mucilage (MU) or calcium phosphate hybrid at pH 8 (MU/HA) and calcium phosphate composites at pH 5 (MU/DCPD) versus MC3T3-E1 cells are presented in Fig. 6. However, the cells treated with free MU did not exhibit significant effect on proliferation activity of MC3T3-E1 cells at 10 and 50 μg/ml, and further increase in the MU concentration results in a mild toxicity (less than 80% compared to the control) over incubation periods. This is possibly due to the affinity of MU to interact with calcium or other ions in the cell culture media, which are vital for cell growth and proliferation. In addition, cell viability can be affected by the physical thickness and hydrophilic nature of MU, as previously reported for alginate [17]. On the other hand, the viability of cells treated with MU/HA even at 200 μg/ml did not have significant effect on the cell over incubation periods. The viability of cells treated with MU/DCPD sample was significantly differed from the viability of MU/HA samples (higher than 80% compared to the control). Data obtained for MU/DCPD hybrid indicated an obvious dose-dependent toxicity. There was a gradual decrease in the viability of MC3T3-E1 cells, with increasing concentrations of MU/DCPD NPs, which presented the highest viability after 1d at concentration 10 μg/ml (112% compared to the control) and the lowest viability (37% compared to the control) after 3 d at concentration 200 μg/ml. It was also observed that a relative increase in the cell viability (more 50%) was seen for MU/DCPD sample after 5d, which could be due to refreshment of cell medium. This behavior indicates that the observed toxicity for MU/DCPD hybrid could be due to the fast resorption of DCPC resulting in acidic condition that limits cell proliferation. Nevertheless, these results suggest that controlling resorption of MU/DCPC hybrid at lower concentrations might be more suitable for the fabrication of inductive biodegradable bone scaffolds in tissue engineering application.
Cell Viability using MTT assay after treatment of MC3T3-E1 cells with serial concentrations (0, 10, 50, 100 and 200 μg ml−1) of neat Mu and the prepared MU-based composites. Data were obtained after 1, 3 and 5 days. (*) means significant difference (P less than 0.05) compared to control, (**) means high significant difference (P more than 0.05) compared to control
Furthermore, inverted light microscopy was used to confirm the cellular proliferation upon the treatment of MC3T3-E1 cells with Mu, Mu/HA and Mu/DCPD composites with a dose of 10 μg ml−1 (Fig. 7). A clear enhancement in the cellular proliferation of osteoblast cell lines upon treatment with Mu/DCPD composites was clearly seen, as compared to the control (Fig. 7A). This is consistent with the MTT assay results shown earlier in Fig. 6. In addition, no significant morphological differences can be observed between the cells treated with prepared composites (Fig. 7).
Conclusion
In the current article, mucilage was extracted from flax seeds and applied as bioactive material for calcium phosphate mineralization. Mucilage was mixed with a solution containing calcium and phosphate ions and adjusted at different pHs. The prepared cross-linked mucilage/calcium phosphate composites at different pHs (5 and 8) showed high degree of homogeneity between the organic and inorganic parts. At pH 5, platelike structures tangled or paralleled were appeared and suggested to be dicalcium phosphate dihydrate. At pH 8, aggregations of nanorods were formed and suggested to be hydroxyapatite. The cell viability studies on cell lines MC3T3-E1 osteoblast cells on hybrid materials showed a clear safety with low toxicity without dose reduction. The mucilage/calcium phosphate composites at different pHs displayed outstanding biocompatibility and therefore could be used for bone regeneration applications.
References
Gupta P, Adhikary M, Kumar M et al (2016) Biomimetic, osteoconductive non-mulberry silk fiber reinforced tricomposite scaffolds for bone tissue engineering. ACS Appl Mater Interfaces 8:30797–30810. https://doi.org/10.1021/acsami.6b11366
Salama A, Shukry N, El-gendy A, El-sakhawy M (2017) Bioactive cellulose grafted soy protein isolate towards biomimetic calcium phosphate mineralization. Ind Crop Prod 95:170–174. https://doi.org/10.1016/j.indcrop.2016.10.019
Costa-Pinto AR, Correlo VM, Sol PC et al (2009) Osteogenic differentiation of human bone marrow mesenchymal stem cells seeded on melt based chitosan scaffolds for bone tissue engineering applications. Biomacromol 10:2067–2073. https://doi.org/10.1021/bm9000102
Bharadwaz A, Jayasuriya AC (2020) Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C 110:110698. https://doi.org/10.1016/j.msec.2020.110698
Tolba E, Wang X, Wang S et al (2020) Amorphous polyphosphate and Ca-carbonate nanoparticles improve the self-healing properties of both technical and medical cements. Biotechnol J 15:2000101. https://doi.org/10.1002/biot.202000101
Nie L, Deng Y, Li P et al (2020) Hydroxyethyl chitosan-reinforced polyvinyl alcohol/biphasic calcium phosphate hydrogels for bone regeneration. ACS Omega 5:10948–10957. https://doi.org/10.1021/acsomega.0c00727
Donnaloja F, Jacchetti E, Soncini M, Raimondi MT (2020) Natural and synthetic polymers for bone scaffolds optimization. Polymers (Basel) 12:905. https://doi.org/10.3390/polym12040905
Porter J, Ruckh T, Popat K (2009) Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Progr 25(6):1539–1560
Huang D, Huang Y, Xiao Y, Yang X, Lin H, Fen G, Zhang X (2019) Viscoelasticity in natural tissues and engineered scaffolds for tissue reconstruction. Acta biomaterialia 97:74–92. https://doi.org/10.1016/j.actbio.2019.08.013
Salama A (2021) Recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int J Biol Macromol 178:240–252. https://doi.org/10.1016/j.ijbiomac.2021.02.143
Salama A, Abou-Zeid RE, Cruz-Maya I, Guarino V (2020) Soy protein hydrolysate grafted cellulose nanofibrils with bioactive signals for bone repair and regeneration. Carbohydr Polym 229:115472. https://doi.org/10.1016/j.carbpol.2019.115472
Salama A, Hesemann P (2020) Synthesis and characterization of N-guanidinium chitosan/silica ionic hybrids as templates for calcium phosphate mineralization. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.01.046
Salama A, El-Sakhawy M (2016) Regenerated cellulose/wool blend enhanced biomimetic hydroxyapatite mineralization. Int J Biol Macromol 92:920–925. https://doi.org/10.1016/j.ijbiomac.2016.07.077
Rao SH, Harini B, Shadamarshan RPK et al (2018) Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int J Biol Macromol 110:88–96. https://doi.org/10.1016/j.ijbiomac.2017.09.029
Kong L, Gao Y, Lu G et al (2006) A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur Polym J 42:3171–3179. https://doi.org/10.1016/j.eurpolymj.2006.08.009
Fedeniuk RW, Biliaderis CG (1994) Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. J Agric Food Chem 42:240–247. https://doi.org/10.1021/jf00038a003
Kreitschitz A (2012) Mucilage formation in selected taxa of the genus Artemisia L. (Asteraceae, Anthemideae). Seed Sci Res 22:177–189. https://doi.org/10.1017/S0960258512000098
Lee K, Kreitschitz A, Lee J et al (2020) Localization of phenolic compounds at an air-solid interface in plant seed mucilage: a strategy to maximize its biological function? ACS Appl Mater Interfaces 12:42531–42536. https://doi.org/10.1021/acsami.0c12357
Dėdinaitė A (2012) Biomimetic lubrication. Soft Matter 8:273–284. https://doi.org/10.1039/C1SM06335A
Mattei L, Di Puccio F, Piccigallo B, Ciulli E (2011) Lubrication and wear modelling of artificial hip joints: a review. Tribol Int 44:532–549. https://doi.org/10.1016/j.triboint.2010.06.010
Boonyagul S, Banlunara W, Sangvanich P, Thunyakitpisal P (2014) Effect of acemannan, an extracted polysaccharide from aloe vera, on BMSCs proliferation, differentiation, extracellular matrix synthesis, mineralization, and bone formation in a tooth extraction model. Odontology 102:310–317. https://doi.org/10.1007/s10266-012-0101-2
Engy M, Abdelhamid SM et al (2020) Manufacture of functional fat-free cream cheese fortified with probiotic bacteria and flaxseed mucilage as a fat replacing agent. Curr Nutr Food Sci 16(9):1393–1403. https://doi.org/10.2174/1573401316666200227112157
Kathyayani D, Mahesh B, Chamaraja N, Madhukar B, Gowda DC (2022) Synthesis and structural characterization of elastin-based polypentapeptide/hydroxypropylmethylcellulose blend films: assessment of miscibility, thermal stability and surface characteristics. Colloids Surf A Physicochem Eng Asp 649:129503. https://doi.org/10.1016/j.colsurfa.2022.129503
Mahesh B, Kathyayani D, Gowda DC, Mrutunjaya K (2020) Blends of synthetic plastic-derived polypeptide with hydroxypropylmethylcellulose and polyvinyl alcohol: unraveling the specific interaction parameters, morphology and thermal stability of the polymers couple. J Polym Res 27:278. https://doi.org/10.1007/s10965-020-02191-5
de Paiva PHEN, Correa LG, Paulo AFS et al (2021) Film production with flaxseed mucilage and polyvinyl alcohol mixtures and evaluation of their properties. J Food Sci Technol 58:3030–3038. https://doi.org/10.1007/s13197-020-04806-7
Ferreira A, Oliveira C, Rocha F (2003) The different phases in the precipitation of dicalcium phosphate dihydrate. J Cryst Growth 252(4):599–611
Mekmene O, Sophie Q, Thierry R et al (2009) Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci Technol 89(3):301–316
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Not applicable.
Author information
Authors and Affiliations
Contributions
AS and ET conceived the work. REA-Z, SS and EMA performed the experiment and preparation section. All authors carried out the analyses, discussion and revisions.
Corresponding authors
Ethics declarations
Conflict of interest
The author declares no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salama, A., Saleh, S., Abou-Zeid, R.E. et al. Flaxseed mucilage/calcium phosphate composites as bioactive material for bone tissue regeneration. Polym. Bull. 80, 13343–13356 (2023). https://doi.org/10.1007/s00289-023-04703-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04703-x