Abstract
In this manuscript, we have demonstrated an efficient and rapid synthetic strategy for preparation of new fluorene–dendron-hybridized blue light-emitting polymers P1–P7 by the reaction of 9,9 long-chain dialkylated fluorenes M2–M8 with dendronized monomer (M1) under microwave-assisted reaction condition. These fluorene–dendron-hybridized polymers P1–P7 were characterized using different spectroscopic techniques. Furthermore, the optophysical properties of these polymers P1–P7 were studied which revealed that these synthesized polymers P1–P7 have potential to emerge as capable materials in the development of diodes, particularly for blue light emission. In the future, similar approaches would be utilized for preparation of light-emitting polymer composite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conjugated polymers can be incorporated in various electronic materials like composites, diodes, transistors, light-emitting diodes [1, 2]. Similar approaches for light-emitting polymers are associated with epoxy materials and its nanocomposites [1,2,3,4,5]. Light-emitting polymers find major applications in large and small area devices such as displays, thin and flat light sources, cloth-type PLEDs, PLEDs in wearable electronics [6].
In the context of ample applications of polymeric materials in diverse fields, the development in the field of polymers is continually progressing [7,8,9,10,11,12]. In the family of conjugated polymers, “dendronized polymers” have embrace reputed space where they are explored as an efficient light-emitting material because of their unique structural features and properties [13,14,15,16,17,18]. There are reports on biopolymer showing good diffraction, absorption, light-emitting properties in the presence and absence of metal nanoparticles [19,20,21,22]. However, they have limited application due to low stability at ambient conditions [23,24,25,26]. Dendronized polymers have compact spherical geometry with three-dimensional tree-like branching arrangement. In the same way, fluorene-based polymers have also emerged as an obvious aspirant in optoelectronics due to their excellent thermal and optical stability. Fluorene-based polymers also possess high fluorescence quantum efficiency, photoluminescence (PL), and electroluminescence efficiency [27,28,29]. Literature review has revealed number of examples of such materials exhibiting hopeful results in electrochromic devices [30,31,32,33,34,35,36,37,38,39,40]. To be particular, the 9,9 long-chain dialkylated fluorenes are very potential candidates in the exploration of blue-emitting materials for PLEDs [41, 42]. Our exhaustive literature study of polymer framework exposed that their properties can tuned through incorporating electron releasing or withdrawing groups in parent fluorene molecule using electrophilic or nucleophilic substitution reactions [43]. In addition to this, fluorene molecule can be structurally modify at 9th position by changing substituent on methylene carbon of fluorene. This fact provides an additional scope for synthesis of fluorene-based molecular framework by substitution on methylene group of the fluorene molecule.
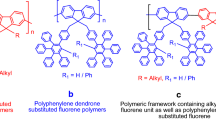
Nowadays, demands for conjugated polymers are increasing drastically due to their potential applications in optoelectronic materials. Hence, there is an essential need to develop the new and rapid method for polymer production enabling its utilization in OLED’s [44, 45]. Literature search showed that Kenneth et.al. [46] have developed method for the synthesis of homopolymers of fluorene with 9,9 dialkyl substitution (Fig. 1A). Similarly, Setayesh et.al have also reported a method for preparation of 9,9 di(polyphenylene)-benzyl)-substituted fluorene homopolymers (Fig. 1B). Due to significant importance of both of these structural units in polymeric framework, it is highly desirable to prepare a new polymer containing both of these units. In this context and continuation of our research work in the synthesis of macromolecular framework [47,48,49,50,51,52], we herein report the nickel (0)-mediated, microwave-assisted polymerization process for synthesis of new fluorene–dendron-hybridized polymers containing 9,9 long-chain dialkylated fluorenes M2–M8 and dendronized monomer (M1) (Fig. 1C). Additionally, we also report the characterization and optophysical study of these newly synthesized polymers.
Experimental
All chemicals were procured from Sigma-Aldrich and S D Fine-Chem, Mumbai. All chemicals were of A.R. grade and used without further treatment. Glasswares were properly washed with deionized water and dried in oven.
Synthesis of polymers
Prepared 20 ml of N,N,dimethyl formamide: toluene (1:4) solvent system to that 0.5 g of bis(1,5-cyclooctadiene)nickel (0), 0.35 g 2,2’-bipyridine, and 0.25 ml cyclooctadiene was added under inert atmosphere. After stirring this mixture, addition of dendronized monomer (M1) 0.250 g and 2,7-dibromo-9,9-diethylfluorene (M2) 0.250 g was accomplished. Then, the reaction vessel was sealed and kept in microwave reactor assembly for 20 min irradiation at 130 °C temperature using 300 W power. The end capping was done by addition of 5 ml of bromobenzene to the reaction mixture. Heating was continued for another 5 min. under identical condition. The above reaction mixture was cooled and diluted by adding 20 ml of dichloromethane and washed with sufficient quantity of deionized water to collect dichloromethane phase which was dried over anhydrous magnesium sulphate (MgSO4) and subjected to evaporation. Before complete drying, it was treated with 20 ml 0.5 M methanolic HCl solution to afford precipitate of the desired fluorene–dendron-hybridized polymers (P1–P7), respectively (all name of polymers are mentioned in supporting information section).
Instrumentation and characterization of synthesized polymers
UV–visible spectroscopic analysis was performed using LAB UV3000plus at ambient temperature with quartz cuvette of 1 cm path-length as a sample holder (200–800 nm). Fluorescence emissions measurement were carried out by RF-5301PC SHIMADZU at ambient temperature with quartz cuvette of 1 × 1 cm path-length (emission at 200 to 800 nm). All cyclic voltametric measurements have been performed using three electrode cell on Electrochemical Work Station, model Autolab PGSTAT 30. Gel permeation chromatograms (GPC) were measured on a PerkinElmer series 200 GPC equipped with an isocratic pump, a solvent degasser, a column oven, a refractive index (RI) detector, and chromatographic column PLgel 10 lm mixed-B, 300 × 7.5. Thermal analysis of polymers was performed with PerkinElmer assembly using 10 °C/min heating rate and nitrogen atmosphere. The X-ray diffraction pattern of the polymers was recorded using Shimadzu maxima 7000 X-ray diffractometer with CuKα radiation (λ = 0.154060 nm) with the scanning range between 5° and 80° to determine its nature (crystalline/amorphous). The surface morphology of polymers was analyzed by SEM. The 1H NMR spectra of the polymers were recorded at 500 mHz using Bruker (Advance) NMR instrument in CDCl3 solvent. Chemical shifts were reported in δ (ppm) values relative to the internal standard tetramethyl silane (TMS). FT-IR spectrum was recorded in the transmittance range of 4000 to 400 cm−1 on PerkinElmer FT-IR spectrometer using Frontier using ATR, and transmittances are reported in cm−1.
Polymer P1 Nature: solid, Color: Green, Yield: 93.10%. 1H NMR (ppm): δ 0.50–1.53 (m, 50H), 3.18 (s, 20H), 6.83–7.82 (m, 360H of aromatic proton) ppm. FT-IR: 2917 cm−1 C–H stretching, 1441 cm−1 C–H bending, 1600 cm−1 aromatic C=C stretching, 3023 cm−1 aromatic C–H stretching, 1495 cm−1 C–C stretching.
Polymer P2 Nature: solid, Color: Brown, Yield: 94.3%. 1H NMR (ppm): δ 0.64–1.93 (m, 84H), 3.14 (s, 24H), 6.81–7.52 (m, 432H of aromatic proton) ppm. FT-IR: (cm−1) = 2927 cm−1 C–H stretching, 1440 cm−1 C–H bending, 1599 cm−1 aromatic C=C stretching, 3024 cm−1 aromatic C–H stretching, 1496 cm−1 C–C stretching.
Polymer P3 Nature: solid, Color: Brown, Yield:95.4%. 1H NMR (ppm): δ 0.58–1.94 (m, 90H), 3.14 (s, 20H), 6.81–7.52 (m, 360H of aromatic proton) ppm. FT-IR: 2926 cm−1 C–H stretching, 1442 cm−1 C–H bending, 1599 cm−1 aromatic C=C stretching, 2980 cm−1 aromatic C–H stretching, 1495 cm−1 C–C stretching.
Polymer P4 Nature: solid, Color: Brown, Yield: 94.3%. 1H NMR (ppm): δ 0.75–1.93 (m, 132H), 3.14 (s, 24H), 6.81–7.49 (m, 432H of aromatic proton) ppm. FT-IR: 2925 cm−1 C–H stretching, 1442 cm−1 C–H bending, 1599 cm−1 aromatic C=C stretching, 3016 cm−1 aromatic C–H stretching, 1496 cm−1 C–C stretching.
Polymer P5 Nature: solid, Color: Light yellow, Yield: 96.7%. 1H NMR (ppm): δ 0.58–1.93 (m, 180H), 3.14 (s, 24H), 6.81–7.53 (m, 432H of aromatic proton) ppm. FT-IR: 2926 cm−1 C–H stretching, 1441 cm−1 C–H bending, 1600 cm−1 aromatic C=C stretching, 3025 cm−1 aromatic C–H stretching, 1497 cm−1 C–C stretching.
Polymer P6 Nature: solid, Color: Brown, Yield: 96.4%. 1H NMR (ppm): δ 0.57–1.93 (m, 204H), 3.39 (s, 24H), 7.25–8.36 (m, 432H of aromatic proton) ppm. FT-IR: 2922 cm−1 C–H stretching, 1441 cm−1 C–H bending, 1599 cm−1 aromatic C=C stretching, 3025 cm−1 aromatic C–H stretching, 1495 cm−1 C–C stretching.
Polymer P7 Nature: solid, Color: Brown, Yield: 93.3%. 1H NMR (ppm): δ 0.89–1.95 (m, 330H of alkyl group), 3.35 (s, 20H of benzyl group), 6.73–7.25 (m, 360H of aromatic proton) ppm. FT-IR: 2921 cm−1 C–H stretching, 1441 cm−1 C–H bending, 1577 cm−1 aromatic C=C stretching, 3024 cm−1 aromatic C–H stretching, 1496 cm−1 C–C stretching.
Results and discussion
Synthesis of fluorene–dendron-hybridized polymers P1–P7
This synthetic approach utilizes microwave as microwave-assisted synthesis methods are rapid and provide uniform heating. This process is also an energy savings with higher yield and cost-effective. Compared to the conventional methods, this method gives small narrow particle size distribution and high purity. The synthesis of 2,7-dibromo-9,9-di(4-pentaphenylphenyl)-benzyl) fluorene (M1) monomer was achieved by using previously published work [47,48,49,50,51,52], and 9,9 long-chain dialkylated fluorene monomers (M2–M8) were synthesized by using method reported by Saikia et al. [39]. The method involves the nucleophilic substitution reaction of 2,7-dibromofluorene with various alkyl bromides under aqueous–organic medium in the presence of TBAI at 100–110 °C for 5 h. Thus, we decided to investigate microwave-assisted polymerization process for the synthesis of fluorene–dendron-hybridized polymers containing 9,9 long-chain dialkylated fluorenes M2–M8 and dendronized monomer (M1) [53,54,55]. We have taken 20 ml of N,N,dimethyl formamide: toluene (1:4) solvent system in which 0.5 g of bis(1,5-cyclooctadiene)nickel (0), 0.35 g 2,2’-bipyridine, and 0.25 ml cyclooctadiene were added under inert atmosphere. After stirring this mixture, addition of dendronized monomer (M1) 0.250 g and 2,7-dibromo-9,9-diethylfluorene (M2) 0.250 g was accomplished. Then, the reaction vessel was sealed and kept in microwave reactor assembly for 20 min, irradiation at 130 °C temperature using 300 W power. The end capping was done by the addition of 5 ml of bromobenzene to reaction mixture and continued the heating for another 5 min under identical parameters. The above mixture was cooled and diluted by adding 20 ml dichloromethane and washed with H2O to collect dichloromethane phase which was dried over anhydrous MgSO4 and evaporated. Before complete drying, it was treated with 20 ml 0.5 M methanolic HCl solution to afford 93.1% yield of green color solid product. The obtained product was extensively characterized by different spectroscopic techniques and gel permeation chromatography (GPC). The characterization data confirmed the formation of desired polymer P1. Further this reaction condition was also tested for polymerization of other monomers M3, M4, M5, M6, M7, M8 and found equally efficient. In this way, we have synthesized fluorene–dendron-hybridized polymers P1–P7 using microwave irradiation with bis (1,5-cyclooctadiene) nickel (0), 2,2’-bipyridyl, cyclooctadiene, in N,N,dimethyl formamide: toluene (1:4) solvent system system at 300 W power at 130 °C for 20 min under inert atmosphere (Fig. 2). Formation of all synthesized polymers P1–P7 was confirmed by various characterization techniques.
Optophysical study of the polymers
Table 1 contains the optophysical study data of fluorene–dendron-hybridized polymers P1–P7 recorded using tetrahydrofuran. Figure 3a, b shows the absorption and photoluminescence spectrums, respectively. In this study, the fluorene–dendron-hybridized polymers P1–P7 exhibited an absorption peak at 344–385 nm that can be assigned to the π → π* transition due to presence of fluorene unit. Furthermore, the observed emission peaks in the range of 436–444 nm in photoluminescence spectrums are a characteristics peak of polymers framework. This optophysical study of the polymers revealed that synthesized series of fluorene–dendron-hybridized polymers, P1–P7, displayed excellent light-emitting property selectively for blue color.
Furthermore, the optical band gap for P1–P7 polymers was derived from the corresponding absorption spectrums and found in 2.99–3.01 eV range, which was in accordance with the reported data related to emission of blue color light [9]. Images of P1–P7 polymers in CHCl3 under visible light and UV light are shown in Fig. 4a, b, respectively. The diluted solutions of the polymer emitted strong blue light under UV excitation compared to visible light. The fluorene–dendron-hybridized P1–P7 polymers not only exhibited good quantum yields but also showed good stoke shift value in 59–96 nm range.
Thermogravimetric analysis (TGA) of synthesized fluorene–dendron-hybridized polymers
We have conducted the thermogravimetric analysis of synthesized fluorene–dendron-hybridized polymers. We have observed the steady weight loss which can be attributed to the decomposition of polymeric framework caused by fragmentation of molecules with breaking of chemical bonds as an effect of increasing temperature [16, 17]. Mentioned in the electronic supplementary information file, displays the thermogram of the thermogravimetric analysis of synthesized fluorene–dendron-hybridized polymers P1–P7 it indicates these polymers underwent multistep decomposition. Specifically, P2, P4, and P5 polymers underwent two-step decomposition, whereas P3, P6, and P7 polymers adapted three step decomposition and P1 polymer followed four-step decomposition process. This thermogravimetric analysis of synthesized fluorene–dendron-hybridized polymers P1–P7 has confirmed the thermal stability with negligible weight loss up to 200 °C.
Cyclic voltammetry (CV) study of the polymers
We have investigated CV studies of the synthesized fluorene–dendron-hybridized polymers P1–P7 in which we observed the irreversible oxidation wave in the range of 0.85–0.98 V of P1–P7 polymers. The obtained data are summarized in Table S1. On the basis of observed onset oxidation potential for the polymers P1–P7, we have approximated the HOMO energy level in − 5.55 to − 5.68 eV range and LUMO energy level in − 2.55 to − 2.67 eV range. In general, all polymers undergo a reversible oxidation process originating from dendron substituted fluorene molecule followed by alkyl substituted fluorene moiety. This study confirms the stability of all polymers in an electrolytic solution (mentioned in the electronic supplementary information file for CV spectrums of polymers P1–P7).
Gel permeation chromatography (GPC) study
GPC is a prominent technique used to establish average molar mass of the copolymers and attests formation of a link between the two constituting blocks. The data obtained from this GPC analysis give molar masses of polymers. GPC analysis was used to obtain the polydispersity index (PDI), degree of polymerization (DP) as well as number average molecular weight (Mn), weight average molecular weight (Mw), size average molecular weight (MZ) of fluorene–dendron-hybridized polymers P1–P7 which are mentioned in Table 2. The data of this study have given PDI values for the polymers in 1.084–4.500 range (Table 2). It is noteworthy to mention that, among the P1–P7 polymers, the polymer which was substituted with dioctyl group (P6) showed higher DP values and the polymer with dipropyl group (P2) showed lower DP values.
Scanning electron microscopy of the polymers
The SEM images showed that the fluorene–dendron-hybridized polymers are microcrystals which have platy rough, rock-shaped, and slightly amorphous globular structure (Fig. 5 mentioned in the electronic supplementary information file). Furthermore, the SEM images do not reveal the separation of phases in polymeric structures. These images confirmed the slight crystalline and amorphous nature of polymers (P1–P7). It was consistent with reported literature data which showed similar platy rough morphology for some blue light-emitting polymers.
X-Ray diffraction study of fluorene–dendron-hybridized polymers P1–P7
Different peak diffraction intensities were observed in obtained XRD diffractograms of fluorene–dendron-hybridized polymers P1–P7 (Fig. 6 and mentioned in the electronic supplementary information file). The range of crystallite size for these polymers was from 0.2 to 6.33 Ǻ (Table S2). The synthesized P2 polymer showed crystalline size of 6.333 Ǻ and 0.215 Ǻ for P6 polymer. Besides this, the rest of the polymers showed crystallite size range from 0.314 to 4.616Ǻ. The diffractograms showed sharp as well as broad peaks; hence, this observation suggested that polymers were slightly crystalline in nature. In the diffractograms of these polymers, broad diffraction peaks were observed which indicated the presence of amorphous content in the polymer chain.
Conclusions
We have developed an efficient synthetic strategy for fluorene–dendron-hybridized polymers P1–P7. Synthesis of these fluorene–dendron-hybridized polymers was achieved by reacting 9,9 long-chain dialkylated fluorenes monomers (M2–M8) with dendronized monomer (M1) under microwave-assisted reaction condition. The structure elucidation and various optoelectronic, thermal, chromatographic, and spectroscopic studies of these polymers were performed by NMR, FT-IR, UV–Vis., GPC, TGA, XRD, and SEM techniques. The optophysical study of these polymers showed well-resolved transitions in the UV–Vis., attributing to the presence of non-breakable polymer linkage, and was found to be stable in THF solvent. The UV–Vis. spectral data were used to calculate direct band gaps of polymers which were observed in 2.99–3.01 eV range. The CV study of fluorene–dendron-hybridized polymers was also performed to get HOMO and LUMO energy-level values. Furthermore, gel permeation chromatography (GPC) study is used to obtain PDI, DP as well as Mn, Mw, MZ for these fluorene–dendron-hybridized polymers P1–P7. Through SEM and X-Ray diffraction study, slight crystalline and amorphous nature of polymers was confirmed. Synthesized polymers have potential to emerge as capable materials in the development of diodes, particularly for blue light emission. In future, we might require use of such polymeric material for preparing light-emitting polymer composites.
References
Li T, Zhang J, Wang H, Hu Z, Yu Y (2013) High-performance light-emitting diodes encapsulated with silica-filled epoxy materials. ACS Appl Mater Interfaces 5(18):8968–8981. https://doi.org/10.1021/am402035r
Huang J-C, Chu Y-P, Wei M, Deanin RD (2004) Deanin, comparison of epoxy resins for applications in light-emitting diodes. Adv Polym Technol 23(4):298–306. https://doi.org/10.1002/adv.20018
Pansare AV, Khairkar SR, Shedge AA, Chhatre SY, Patil VR, Nagarkar AA (2018) In situ nanoparticle embedding for authentication of epoxy composites. Adv Mater 30(33):1801523. https://doi.org/10.1002/adma.201801523
Tan EKW, Shrestha PK, Pansare AV, Chakrabarti S, Li S, Chu D, Lowe CR, Nagarkar AA (2019) Density modulation of embedded nanoparticles via spatial, temporal, and chemical control elements. Adv Mater 31(51):1901802. https://doi.org/10.1002/adma.201901802
Pansare AV, Chhatre SY, Khairkar SR, Bell JG, Barbezat M, Chakrabarti S, Nagarkar AA, Appl ACS (2020) “Shape-Coding”: morphology-based information system for polymers and composites. Mater Interfaces 12(24):27555–27561. https://doi.org/10.1021/acsami.0c05314
Babu DP, Kumar SN, Kumar NS, Naidu KCB, Basha DB (2020) Applications of polymer light-emitting devices and displays, polymers for light-emitting devices and displays. Scrivener Publishing LLC. https://doi.org/10.1002/9781119654643.ch1
Hains AW, Liang Z, Woodhouse MA, Gregg BA (2010) Molecular semiconductors in organic photovoltaic cells. Chem Rev 110:6689–6735
Jiang L, Dong H, Hu W (2010) Organic single crystal field-effect transistors: advances and perspectives. J Mater Chem 20:4994–5007
Grimsdale AC, Chan KL, Martin RE (2009) Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem Rev 109:897–1091
Cho HJ, Jung BJ, Cho NS (2003) Synthesis and characterization of thermally stable blue light-emitting polyfluorenes containing siloxane bridges. Macromolecules 36:6704–6710
Wang YJ, Yu G (2019) Conjugated polymers: from synthesis, transport properties, to device applications. J Polym Sci Pol Phys 57:1557–1558
Morin PO, Bura T, Leclerc M (2016) Realizing the full potential of conjugated polymers: innovation in polymer synthesis. Mater Horiz 3:11–20
Beaujuge PM, Reynolds JR (2010) Color control in pi-conjugated organic polymers for use in electrochromic devices. Chem Rev 110:268–320
Klauk H (2010) Organic thin-film transistors. Chem Soc Rev 39:2643–2666
Burroughes JH, Bradley DDC, Brown AR, Marks RN, Mackay K, Friend RH, Burn PL, Holmes AB (1990) Light-emitting diodes based on conjugated polymers. Nature 347:539–541
Tomalia DA, Frechet JMJ (2002) Discovery of dendrimers and dendritic polymers: a brief historical perspective. J Polym Sci Part A Polym Chem 40:2719–2728
Hawker CJ, Frechet JMJ (1992) The synthesis and polymerization of a hyper branched polyether macromonomer. Polymer 33:1507–1511
Percec V, Heck J, Tomazos D, Falkenberg F, Blackwell H, Ungar G (1993) Self-assembly of taper-shaped monoesters of oligo(ethylene oxide) with 3,4,5-tris(p-dodecyloxybenzyloxy)benzoic acid and of their polymethacrylates into tubular supramolecular architectures displaying a columnar mesophase. J Chem Soc 1:2799–2811
Pansare AV, Kulal DK, Shedge AA, Patil VR (2016) Green synthesis of anticancerous honeycomb PtNPs clusters: their alteration effect on BSA and HsDNA using fluorescence probe. J Photochem Photobiol B Biol 162:473–485. https://doi.org/10.1016/j.jphotobiol.2016.07.021
Pansare AV, Kulal DK, Shedge AA, Patil VR (2016) hsDNA groove binding, photocatalytic activity, and in vitro breast and colon cancer cell reducing function of greener SeNPs. Dalton Trans 45:12144–12155. https://doi.org/10.1039/C6DT01457G
Pansare AV, Shedge AA, Sonawale MC, Pansare SV, Mahakal AD, Khairkar SR, Chhatre SY, Kulal DK, Patil VR (2022) Deciphering the sensing of α-amyrin acetate with hs-DNA: a multipronged biological probe. RSC Adv 12:1238. https://doi.org/10.1039/D1RA07195E
Shedge AA, Pansare SV, Khairkar SR, Chhatre SY, Chakrabarti S, Nagarkar AA, Pansare AV, Patil VR (2020) Nanocomposite of functional silver metal containing curcumin biomolecule model systems: protein BSA bioavailability. J Inorg Biochem 212:111210. https://doi.org/10.1016/j.jinorgbio.2020.111210
Pansare AV, Pansare PV, Shedge AA, Pansare SV, Patil VR, Terrasi GP (2022) Click gold quantum dots biosynthesis with conjugation of quercetin for adenocarcinoma exertion. RSC Adv 12:18425. https://doi.org/10.1039/D2RA02529A
Pansare AV, Shedge AA, Chhatre SY, Das D, Murkute P, Pansare SV, Nagarkar AA, Patil VR, Chakrabarti S (2019) AgQDs employing black box synthetic strategy: photocatalytic and biological behavior. J Lumin 212:133–140. https://doi.org/10.1016/j.jlumin.2019.04.014
Pansare AV, Shedge AA, Patil VR (2018) Discrete SeNPs-macromolecule binding manipulated by hydrophilic interaction. Int J Biol Macromol 107:1982–1987. https://doi.org/10.1016/j.ijbiomac.2017.10.065
Pansare AV, Pansare SV, Pansare PV, More BP, Nagarkar AA, Barbezat M, Donde KJ, Patil VR, Terrasi GP (2022) Economical gold recovery cycle from bio-sensing AuNPs: an application for nanowaste and COVID-19 testing kits. Dalton Trans 51:14686–14699. https://doi.org/10.1039/D2DT01405J
Peng Q, Huang Y, Cao Y (2004) Synthesis and characterization of new red-emitting polyfluorene derivatives containing electron-deficient 2-Pyran-4-ylidene−malononitrile moieties. Macromolecules 37:260–266
Ranger M, Rondeau D, Leclerc M (1997) New well-defined Poly(2,7-fluorene) derivatives: photoluminescence and base doping. Macromolecules 30:7686–7691
Grisorio R, Suranna GP, Mastrorilli P (2007) Insight into the role of oxidation in the thermally induced green band in fluorene based systems. Adv Funct Mater 17:538–548
Sandee AJ, Williams CK, Evans NR, Davies JE, Boothby CE, Kohler A, Friend RH, Holmes AB (2004) Solution-processible conjugated electrophosphorescent polymers. J Am Chem Soc 126:7041–7048
Pinner DJ, Friend RH, Tessler N (1999) Transient electroluminescence of polymer light emitting diodes using electrical pulses. J Appl Phys 86:5116–5130
Charas A, Morgado J, Martinho JMG, Alcacer LS, Lim F, Friend RH, Cacialli F (2003) Synthesis and luminescence properties of three novel polyfluorene copolymers. Polymer 44:1843–1850
Bezgin B, Cihaner A, Onal AM (2008) Electrochemical polymerization of 9-fluorenecarboxylic acid and its electrochromic device application. Thin Solid Films 516:7329–7334
Tsuie B, Reddinger JL, Sotzing GA, Soloducho J, Katritzky AR, Reynolds JR (1999) Electroactive and luminescent polymers: new fluorene-heterocycle-based hybrids. J Mater Chem 9:2189–2200
Çarbas BB, Kivrak A, Onal AM (2012) A new processable electrochromic polymer based on an electron deficient fluorene derivative with a high coloration efficiency. Electrochim Acta 58:223–234
Nie G, Yang H, Chen J, Bai Z (2012) Novel high-quality electrochromic material from 3,4-ethylenedioxythiophene bis-substituted fluorine. Org Electron Phys Mater Appl 13:2167–2176
Liu J, Tu G, Zhou Q, Cheng Y, Geng Y, Wang L, Ma D, Jing X, Wang FJ (2006) Highly efficient green light emitting polyfluorene incorporated with 4-diphenylamino-1,8-naphthalimide as green dopant. J Mater Chem 16:1431–1438
Evans NR, Devi LS, Mak CSK, Watkins SE, Pascu SI, Kohler A, Friend RH, Williams CK, Holmes AB (2006) Triplet energy back transfer in conjugated polymers with pendant phosphorescent iridium complexes. J Am Chem Soc 128:6647–6656
Saikia G, Iyer PK (2010) Facile C-H alkylation in water: enabling defect-free materials for optoelectronic devices. J Org Chem 75:2714–2717
Setayesh S, Grimsdale AC, Weil T, Enkelmann V, Mullen K, Meghdadi F, List EJ, Leising GJ (2001) Polyfluorenes with polyphenylene dendron side chains: toward non-aggregating, light-emitting polymers. J Am Chem Soc 123:946–953
Leclerc MJ (2001) Polyfluorenes: twenty years of progress. Polym Sci Part A Polym Chem 39:2867–2873
Neher D (2001) Polyfluorene homopolymers: conjugated liquid crystalline polymers for bright blue emission and polarized electroluminescence. Macromol Rapid Commun 22:1365–1385
Woo EP, Shiang WR, Inbasekaran M, Roof GR, Bernius MT, Weishi W (2005) Fluorene-containing polymers and compounds useful in the preparation thereof. US Patent 6900285. 1–30
Williams JAG (2007) “Organic light-emitting devices: synthesis, properties and applications.” Platinum Metals Rev 51:85–86
Newkome GR, Moorefield CN, Vögtle F, Vögtle F, Vögtle F, Chemist G (2001). In: Moorefield CN, Vogtle F (eds) Dendrimers and dendrons: concepts syntheses, applications. Wiley, New York, pp 1–623
Kenneth RC (2002) Nickel(0) mediated coupling polymerizations via microwave-assisted chemistry. Macromolecules 35:6757–6759
Barve KA, Raut SS, Mishra AV, Patil VR (2011) Synthesis and studies of blue light emitting polymers containing triphenylamine-substituted fluorene and diphenylanthracene moiety. J Appl Polym Sci 122:3483–3492
Raut SS, Patil VR (2013) Synthesis and spectral studies of 6, 13-di (p-hydroxyphenyl) pentacene and 6, 13-di (p-hydroxynapthyl) pentacene. Polycycl Aromat Comp 33:127–137
Kadu RK, Patil VR (2017) New strategy for synthesis of polyphenylene substituted dendronized monomers containing fluorene unit and the study of their properties. Polycycl Aromat Comp 37:395–406
Chalke RM, Patil VR (2017) New approaches towards the synthesis and characterization of alkoxy substituted spirobifluorenes and spirosilabifluorenes for organic optoelectronics. J Macromol Sci A 54:556–534
Chalke RM, Patil VR (2017) Novel methoxy spirobifluorene and alkyl substituted diphenylacene based organic blue light emitting polymers for application in organic electronics. Polymer 123:355–365
Kadu RK, Thakur PB, Patil VR (2018) Photophysical properties of new fuorene-based conjugated polymers containing polyphenylene-substituted dendronized core. Polym Bull. https://doi.org/10.1007/s00289-018-2401-3
Shinpei MY, Susumu T, Junichi S, Kenji M, Shunzo S, Kenji T (2009) Microwave-assisted preparation of Poly(fluorene)s by ni-catalyzed polymerization. Polym J 4:327–331
Matthias B, Luisa D, Bozano J, Campbell S, Kenneth RC (2005) Design and synthesis of new polymeric materials for organic nonvolatile electrical bistable storage devices: poly(biphenylmethylene)s. Macromolecules 38:4147–4156
Yoan CS, Joseph JP, Christine M, Kenneth RC, Bryan CE (2009) Synthesis of polyfluorenes with pendant silylcarboranes. Macromolecules 42:512–516
Acknowledgements
A.V.P. acknowledges Composites Group, Mechanical Systems Engineering, Swiss Federal Laboratories for Materials Science and Technology-Empa, 8600 Dübendorf, Switzerland.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadu, R.K., Thakur, P.B., Zote, S.W. et al. Blue light-emitting fluorene–dendron hybridized polymers: optophysical features. Polym. Bull. 80, 10379–10392 (2023). https://doi.org/10.1007/s00289-022-04571-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04571-x