Abstract
Ionic hydrogels are favorable for drug delivery applications, and fluorescence probes have been employed to analyze drug carriers. In this work, we studied swelling properties of pH-sensitive poly (acrylic acid-co-acrylamide) P(AAc-co-AAm) hydrogels in the presence of 30 vol% AAc at different pH values by steady-state fluorescence technique. We introduced a hydrophilic fluorescence probe, pyranine 4 (4sPy), due to its emission spectrum independent of pH. 4sPy molecules were trapped in the P(AAc-co-AAm) hydrogel network during the gelation before the swelling process. We monitored the effect of pH on fluorescence intensities of 4sPy during in situ swelling processes and also compared the behavior of trapped 4sPy molecules with the behavior of trapped drug carriers in a controlled drug delivery system. Most of the 4sPy molecules were released out from the hydrogels at high pH, such as drug molecules in the drug delivery system. Our results indicated that these hydrogels reached the maximum swelling ratio at pH = 9. The cooperative diffusion coefficient, D, was found using in situ fluorescence and gravimetric results as nearly 10−8 m2/s for different pH values that are comparable to the diffusion coefficient of drug molecules in drug delivery systems in the literature. Scanning electron microscope results indicated the change in morphology of anionic hydrogels swollen at different pH values. These results showed that P(AAc-co-AAm) hydrogels could be applicable for jejunum targeted drug delivery systems, and the 4sPy can be a candidate probe to monitor the drug carriers.







Similar content being viewed by others
References
Koetting MC, Peters JT, Steichen SD, Peppas NA (2015) Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater Sci Eng R 93:1–49. https://doi.org/10.1016/j.mser.2015.04.001
Van der Linden HJ, Herber S, Olthuis W, Bergveld P (2003) Stimulus-sensitive hydrogels and their applications in chemical (micro)analysis. Analyst 128:325–331. https://doi.org/10.1039/b210140h
Osada Y, Kajiwara K (2001) Gels handbook. Academic Press, San Diego. https://doi.org/10.1016/B978-0-12-394690-4.X5073-7
Jia HZ, Zhu JY, Wang XL, Cheng H, Chen G, Zhao YF, Zeng X, Feng J, Zhang XZ, Zhuo RX (2014) A boronate-linked linear-hyperbranched polymeric nano vehicle for pH-dependent tumor-targeted drug delivery. Biomaterials 35:5240–5249. https://doi.org/10.1016/j.biomaterials.2014.03.029
Jiang F, Chen S, Cao Z, Wang G (2016) A photo, temperature, and pH-responsive spiropyran-functionalized polymer: synthesis, self-assembly and controlled release. Polymer 83:85–91. https://doi.org/10.1016/j.polymer.2015.12.027
Hibbins AR, Kumar P, Choonara YE, Kondiah PD, Marimuthu T, Toit LCD, Pillay V (2017) Design of a versatile pH-responsive hydrogel for potential oral delivery of gastric-sensitive bioactives. Polymers 9:474–492. https://doi.org/10.3390/polym9100474
Kroh C, Wuchrer R, Günther M, Härtling T, Gerlach G (2018) Evaluation of the pH-sensitive swelling of a hydrogel by means of a plasmonic sensor substrate. J Sens Sens Syst 7:51–55. https://doi.org/10.5194/jsss-7-51-2018
Gupta P, Vermani K, Garg S (2002) Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discovery Today 7:569–579. https://doi.org/10.1016/s1359-6446(02)02255-9
Rizwan M, Yahya R, Hassan A, Yar M, Azzahari AD, Selvanathan V, Sonsudin F, Abouloula CN (2017) pH-sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 9:137–174. https://doi.org/10.3390/polym9040137
Şolpan D, Duran S, Saraydin D, Güven O (2003) Adsorption of methyl violet in aqueous solutions by poly(acrylamide-co-acrylic acid) hydrogels. Radiat Phys Chem 66:117–127. https://doi.org/10.1016/s0969-806x(02)00384-5
Vernon B, Kim S, Bae Y (1999) Insulin release from islets of Langerhans entrapped in a poly(N-isopropyl acrylamide-co-acrylic acid) polymer gel. J Biomater Sci Polym Ed 10:183–198. https://doi.org/10.1163/156856299x00126
Mutalabisin M, Chatterjee B, Jaffri J (2018) pH-responsive polymers in drug delivery. Res J Pharm Technol 11:5115–5122. https://doi.org/10.5958/0974-360x.2018.00934.4
Bolla P, Rodriguez V, Kalhapure R, Kolli C, Andrews S, Renukuntla J (2018) A review on pH and temperature-responsive gels and other less explored drug delivery systems. J Drug Delivery Sci Technol 46:416–435. https://doi.org/10.1016/j.jddst.2018.05.037
Zhou X, Weng L, Chen Q, Zhang J, Shen D, Li Z, Shao M, Xu J (2003) Investigation of pH sensitivity of poly(acrylic acid-co-acrylamide) hydrogel. Polym Int 52:1153–1157. https://doi.org/10.1002/pi.1207
Nath J, Chowdhury A, Ali I, Dolui SW (2019) Development of a gelatin-g-poly(acrylic acid-co-acrylamide)–montmorillonite superabsorbent hydrogels for in vitro controlled release of vitamin B12. J Appl Polym Sci 136:47596–47607. https://doi.org/10.1002/app.47596
Xu L, Qiu L, Sheng Y, Sun Y, Deng L, Li X, Bradley M, Zhang R (2018) Biodegradable pH-responsive hydrogels for controlled dual-drug release. J Mater Chem B 6:510–517. https://doi.org/10.1039/C7TB01851G
Kim D, Park K (2004) Swelling and mechanical properties of super porous hydrogels of poly(acrylamide-co-acrylic acid)/polyethyleneimine interpenetrating polymer networks. Polymer 45:189–196. https://doi.org/10.1016/j.polymer.2003.10.047
Zhang JP, Li A, Wang AQ (2005) Study on superabsorbent composite. V. Synthesis, swelling behaviors, and application of poly(acrylic acid-co-acrylamide)/sodium humate/attapulgite superabsorbent composite. Polym Adv Technol 16:813–820. https://doi.org/10.1002/pat.657
Thakur A, Wanchoo RK, Singh P (2011) Structural parameters and swelling behavior of pH-sensitive poly(acrylamide-co-acrylic acid) hydrogels. Chem Biochem Eng Q 25:181–194. https://doi.org/10.15255/CABEQ.2014.187
Qu J, Zhao X, Ma PX, Guo B (2017) pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater 58:168–180. https://doi.org/10.1016/j.actbio.2017.06.001
Qu J, Zhao X, Ma PX, Guo B (2018) Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized smart drug release. Acta Biomater 72:55–69. https://doi.org/10.1016/j.actbio.2018.03.018
Liang Y, Zhao X, Ma PX, Gou B, Du Y, Han X (2019) pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan grafted dihydocaffeic acid and oxidized pullulan for localized drug delivery. J Colloid Interface Sci 15:224–234. https://doi.org/10.1016/j.jcis.2018.10.056
Ray D, Mohapatra DK, Mohapatra RK, Mohanta GP, Sahoo PK (2008) Synthesis and colon-specific drug delivery of a poly(acrylic acid-co-acrylamide)/MBA nanosized hydrogel. J Biomater Sci Polym Ed 19:1487–1502. https://doi.org/10.1163/156856208786140382
Singh B, Bala R, Chauhan N (2008) In vitro release dynamics of model drugs from psyllium and acrylic acid-based hydrogels for the use in colon-specific drug delivery. J Mater Sci Mater Med 19:2771–2780. https://doi.org/10.1007/s10856-008-3406-5
Anwar M, Pervaiz F, Shoukat H, Noreen S, Shabbir K, Majeed A, Ijaz S (2021) Formulation and evaluation of an interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym Bull 78:59–80. https://doi.org/10.1007/s00289-019-03092-4
Tian B, Liu S, Wu S, Lu W, Wang D, Jin L, Hu B, Li K, Wang Z, Quan Z (2017) pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf B 154:287–296. https://doi.org/10.1016/j.colsurfb.2017.03.024
Valeur B, Berberan-Santos MN (2012) Molecular fluorescence: principles and applications, 2nd edn. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. https://doi.org/10.1002/9783527650002
Jameson DM (2014) Introduction to Fluorescence. CRC Press, Boca Raton. https://doi.org/10.1021/b16502
Aktaş DK, Evingür GA, Pekcan Ö (2007) Study on swelling of hydrogels (PAAm) at various temperatures using fluorescence technique. J Mater Sci 42:8481–8488. https://doi.org/10.1007/s10853-007-1764-x
Aktaş DK, Evingür GA, Pekcan Ö (2009) A fluorescence study on the swelling of hydrogels (PAAm) at various cross-linker contents. Adv Polym Technol 28:215–223. https://doi.org/10.1002/adv.20163
Kaya D, Pekcan Ö, Yılmaz Y (2004) Direct test of the critical exponents at the sol-gel transition. Phys Rev E 69:016117–016127. https://doi.org/10.1103/physreve.69.016117
Yılmaz Y, Uysal N, Gelir A, Güney O, Aktaş DK, Göğebakan S, Öner A (2009) Elucidation of multiple-point interactions of pyranine fluoroprobe during the gelation. Spectrochim Acta Part A Mol Biomol Spectrosc 72:332–338. https://doi.org/10.1016/j.saa.2008.09.012
Shibayama M, Tanaka T (2005) Volume phase transition and related phenomena of polymer gels. Adv Polym Sci 109:1–62. https://doi.org/10.1007/3-540-56791-7_1
Bartil T, Bounekhel M, Cedric C, Jeerome R (2007) Swelling behavior and release properties of pH-sensitive hydrogels based on methacrylic derivatives. Acta Pharm 57:301–314. https://doi.org/10.2478/v10007-007-0024-6
Di Cagno MP, Clarelli F, Våbenø J, Lesley C, Rahman SD, Cauzzo J, Franceschinis E, Realdon N, Stein PC (2018) Experimental determination of drug diffusion coefficients in unstirred aqueous environments by temporally resolved concentration measurements. Mol Pharm 15:1488–1494. https://doi.org/10.1021/acs.molpharmaceut.7b01053
Hayashi K, Mitsuyoshi Y, Kamei T, Shimanouchi T, Suga K, Okamoto Y, Nakamura H, Umakoshi H (2018) Design of pyrene-fatty acid conjugates for real-time monitoring of drug delivery and controllability of drug release. ACS Omega 3:3572–3580. https://doi.org/10.1021/acsomega.7b02061
Acknowledgements
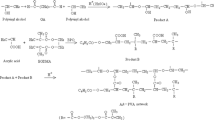
This work was supported by Istanbul Technical University (ITU) under grand TGA-2018-41020. We would like to thank Biorender.com, which provided Fig. 6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aktaş, D.K., Öztekin, F. pH-Sensitive poly (acrylic acid-co-acrylamide) anionic hydrogels for jejunum targeted drug delivery systems. Polym. Bull. 80, 2801–2813 (2023). https://doi.org/10.1007/s00289-022-04188-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04188-0