Abstract
Chitosan biological macromolecule is a versatile polymer; chemical modification has been carried out that lead to the formation of chitosan grafted polymers composites (Chito-g-PC). We proposed synthesis of six various Chito-g-PC as sorbents for toxic dyes. A novel graft copolymerization method based on radical polymerization with vinyl monomer like acrylic acid, acrylamide, N-isopropylacrylamide, methacrylic acid and polyacrylonitrile were utilized in order to address the large amount of swelling at four different pH buffers solution. The effect of initiator and monomer concentration, time and temperature on % grafting and % grafting efficiency were performed. Comparative characterization of Chito and Chito-g-PC were evaluated by SEM, XRD and FTIR, as well as solubility characteristics of the composites were determined by various pH buffer solution. Cationic toxic dyes Malachite green (MG) and Methylene blue (MB) were selected as the sorbet, and Chito-g-PC were used as biosorbents. Thermodynamic analysis showed that the sorption process was spontaneous and endothermic with an increased randomness. The sorption experiments were realized with six different Chito-g-PC for MG and MB at various pH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently various biosorbent materials such as fungal or bacterial biomass and biopolymers are obtained from the natural resources in large quantities [1, 2]. Special attention has been given to polysaccharides such as chitosan, a natural amino polymer [3]. It is clear from the literature that the bio sorption of dyes using chitosan is one of the more frequently reported emerging methods for the removal of pollutants. Chitosan has been investigated by several researchers as a bio sorbent for the capture of dissolved dyes from aqueous solutions [4]. This natural polymer possesses several intrinsic characteristics that make it an effective biosorbent for the removal of color compound. Its use as a bio sorbent is justified by two important advantages: firstly, its low cost compared to commercial activated carbon, and secondly chitosan, derived by deacetylation of the naturally occurring biopolymer chitin, which is the second most abundant polysaccharide after cellulose [5,6,7].
Dyes are ionic, aromatic organic compounds with structures including aryl rings which have delocalized electron systems. The color of a dye is provided by the presence of a chromophore group. The presence of ionizing groups known as auxochromes results in a much stronger alteration of the maximum absorption of the compound and provides a bonding affinity. Some common auxochrome groups include –NH3, –COOH, –HSO3 and –OH [8,9,10].
Chitosan is an amenable molecule and without disturbing degree of polymerization, one can chemically modify this acquiescent polymer, since it provides functional groups as primary amine (–NH2 group of C2) and primary (–OH group of C6) as well as a secondary (–OH group of C3) hydroxyl groups in its monomers. Hence there are three main probable positions for chemical grafting.
Chitosan-based adsorbents have received a lot of attention for adsorption of dyes. Various modifications of this polysaccharide have been investigated to improve the adsorption properties as well as mechanical and physical characteristics of chitosan. In this paper we discuss chitosan and its Chito-g-PC for application in the removal of dyes from water. Modification of chitosan changes the original properties of this material so that it can be more suitable for adsorption of different types of dyes. Many chitosan derivatives have been obtained through chemical and physical modifications of raw chitosan that includes cross-linking, grafting and impregnation of the chitosan backbone [11,12,13]. A chemical modification opens way to utilization of chitosan. The grafted composites have biocompatibility, biodegradability and non-carcinogenicity [14]. Keeping in view modified properties of chitosan grafted composite it was thought to study application of prepared composites. Almost all composite have excellent solvent absorptivity due to interstitial spaces and high lipophilicity [15,16,17]. This property may be utilized for various technical application and drug delivery. The composite have affinity for few metal ions, hence it is studied for removal of toxic metal ion from water samples [18]. In addition to high absorptivity and adsorption tendency for heavy metal ion, the composites are more suitable for removing of poisonous dyes, which are constantly discharged to water and soil from textile and dyes industries, tanneries, solid polymer electrolytes and electroplating industries. [19,20,21,22,23].
The adsorption capacity of two water-soluble toxic cationic dyes MB and MG have been reported in this research using Chito-g-PC. The swelling ratio and pH dependent study of Chito-g-PC have also been studied.
Materials and method
Chitosan (from Sigma Aldrich Mol wt. 22.742 kDa and degree of deacetylation of 75%), Acrylic acid (AA, Merck) and acrylamide (AM, Merck) N-isopropylacrylamide, methacrylic acid, acrylonitrile, were used after vacuum distillation. Ammonium persulfate (APS, Merck) was used without purification. Methylene bisacrylamide (MBA, Fluka) was used as received.
Polymer composite preparation
Chito-g-poly (acrylic acid–co-acrylamide), Chito-g-polyacrylamide, Chito-g-polyacrylicacid, Chito-g-N-isopropyl acrylamide, Chito-g-poly(methacrylic acid) and Chito-g-Polyacrylonitrile composite were synthesized by free radical mechanisms method by using ammonium per sulphate as indicator [24, 25]. One representative synthesis of Chito-g-poly (acrylic acid–co-acrylamide) is given here in detail (see Figs. 1 and S1).
Chitosan was dissolved in degassed distilled water containing 1% of acetic acid. 0.50 g of chitosan was dissolved in 30.0 ml of distilled 1% acetic acid solution. The reactor was placed in a water bath preset at 60 °C, then 0.10 g of potassium per sulphate (KPS) as an initiator was added to chitosan solution and was allowed to stir for 10 min at 60 °C. After adding KPS, 0.5 ml of acrylic acid and 0.5 g of acrylamide were added simultaneously to the chitosan solution. MBA solution (0.1 g in 5 ml H2O) was added to the reaction mixture after addition of monomers and mixture was continuously stirred (600 rpm) for 1.0 h under nitrogen atmosphere Figure S2 [24].
The swelling properties as well as dyes extraction properties of composites have been studied by preparing synthetic solutions of Chito-g-PC. All samples and stock solution were prepared in distilled water.
The composite with acrylic acid-co-acrylamide, acrylamide, N-isopropylacrylamide were cross-linked with MBA, whereas in the remaining three composites with monomer i.e., acrylic acid, methylmethacrylic acid and acrylonitrile cross linker MBA was not used (Figure S1). The names of composites and their abbreviations shown in Table S1.
Polymer composites characterization
The structural parameter for Chito-g-PC was further investigated by using Fourier-transform infrared spectroscopy (FTIR), Differential scanning calorimetry (DSC), X-ray diffraction (XRD) and Scanning Electron Microscopy (SEM). The IR spectrum of pure chitosan was used for comparing Chito-g-PC prepared by grafting with various vinyl monomers. In DSC technique amount of heat required to increase temperature of sample and references were measured as a function of temperature. Both sample and reference maintained at nearly same temperature throughout experiment. The glass transition temperature (Tg) of Chito-g-PC were measured by DSC. The change in the chitosan structure after Chito-g-PC were investigated by XRD. The surface morphology and cross section morphology of chitosan and Chito-g-PC were characterized by SEM.
Swelling study of polymer composites
A Chito-g-PC sample (0.10 g) was weighed and transferred into tea bag and immersed in 100 ml distilled water and allowed to soak for 2 hrs at room temperature. The equilibrated swollen composite was allowed to drain by removing the tea bag from water and hanging until no drop drained (20 min). The bag was then weighed to determine the weight of the swollen Chito-g-PC. The equilibrium swelling was calculated using the following equation [26].
Swelling ratio (absorbency) was calculated as grams of water per gram of resin (g/g). The accuracy of the measurements was ±3%. Buffer solution of pH 3, 5, 9 and 11 were prepared by using respective buffer tablets (Table S2). Dry weight of Chito-g-PC were measured and inserted into respective buffer solution and then weight of Chito-g-PC were measured after 2 hrs at ambient temperature and swelling ratio of following Chito-g-PC solution were observed (Fig. 2).
Adsorption studies of polymer composites
A stock solution of MB and MG dyes (1.0 g/L) was prepared by dissolving 1 g of MB and MG dyes powder in 1 L of double distilled water. The desired concentrations ranging from 10 to 60 mg/L were obtained by dilution. For each adsorption experiment, 50 ml of the dye solution with a specified concentration was stirred at 100 rpm in a glass flask. The pH of solutions was adjusted to a desired value by adding 0.1 mol/L NaOH or HCl solution. 50 mg (Chitosan-g-poly acrylic acid-co-acrylamide) composite samples were put into conical flasks, into which 50 mL aqueous solutions of MB and MG were added separately and vibrated at 125 rpm in a shaking water bath. When the adsorption reached equilibrium after 12 h, the conical flasks were taken out and centrifuged to separate the composite and the solution. The concentrations of the free dyes in the filtrate were analyzed with a UV–Vis spectrophotometer Lambda 650 (PerkinElmer). In this way batch extraction study were carried out for all chitosan grafted composites i.e., Chitosan-g-polyacrylamide, Chitosan-g-polyacrylic acid, Chitosan-g-N-isopropylacrylamide, chitosan-g-poly (methacrylic acid) copolymer and Chitosan-g-Polyacrylonitrile by varying pH.
Result and discussion
The objective of the present work was to modify chitosan with selected vinyl monomers through free radical graft copolymerization for the development of synthetic natural polymer hybrid materials with defined structures, compositions and tailor-made properties. An elaborate physicochemical, thermo-analytical and morphological evaluation of the copolymers were performed using FTIR, DSC, XRD and mechanical property evaluation, swelling studies, SEM studies etc. As Chitosan is second most abundant polysaccharide on earth which gives a green route for metal extraction. The study throws some light on the prospects of synthetic protocols that can be adopted to realize chitosan-g-vinyl polymers with controlled extent of grafting and nature of graft. As a general conclusion, the objective of the study to synthesize natural–synthetic hybrid materials with tailor-made properties has been achieved through the copolymerisation of chitosan with the selected vinyl monomers like AA, AM, NIPAAM, MA and PAN.
Polymer composites characterization
FTIR studies
The main characteristic peaks in chitosan spectrum were observed at 3455 cm−1 (O–H stretch, N–H stretch, overlapped), 2922 cm−1 and 2871 cm−1 (C–H stretch), 1656 cm−1(NH2 deformation), 1620 cm−1 (N–H bend), 1327 cm−1 (C–N stretch), 1155 cm−1 (bridge –O– stretch), and 1092 cm−1 (C–O stretch). In the spectrum of grafted chitosan, addition to the chitosan characteristic peaks, some new absorption peaks were appeared (Figure S3).
Chito-g-AA-co-AM composite showed broad band at 3342 cm−1 which corresponds to O–H and amide (-NH) groups stretching. The small peak at 2921 cm−1 accredited to the asymmetric stretching frequency of -CH group, while peak at 1658 cm−1 was assigned for N–H deformation bending of backbone (Figure S4). A very intense characteristic band at 1665 cm−1 was observed due to C=O asymmetric stretching in carboxylate anion and was reconfirmed by peak at 1449 cm−1. Chito-g-AA-co-AM, showed two additional peaks at 1726 cm−1 (carboxylic acid) and 1564 cm−1 (amide) which were absent in the IR spectrum of pure chitosan, this clearly indicates the formation of graft copolymer [27]. FTIR spectrum of Chito-g-AM composite showed two peaks at 3206 cm−1 and 1660 cm−1 corresponding to the primary amides and amide –NH stretching vibrations. Polyacrylamide can be identified by its characteristic amide C=O peak at 1660 cm−1, which is also present in the spectrum of Chito-g-PAM. Chito-g-AA—The peak at 1735 cm−1 corresponds to the carboxyl absorption from grafted acrylic acid and the peaks at 821 cm−1 and 619 cm−1 were characteristic absorptions of acrylic acid. Chito-g-NIPAAM—The FTIR spectrum of chitosan with a 75% deacetylation degree indicated that peaks appeared at 3436 cm−1 (corresponding to OH stretching vibration, which indicated a chemical reaction between chitosan and N-isopropylacrylamide), 1658 cm−1, and 1569 cm−1 could be assigned to a hydroxyl group, carbonyl stretching vibration (amide I), and N–H bending vibration (amide II) of a primary amino group, respectively. Chito-g-PMMA- The intense band at 3423 cm−1 was assigned to the stretching vibrations of O–H group. The absorption bands at 1154 cm−1 and 1033 cm−1 were assigned for asymmetric stretching of the C–O–C bridge. Chito-g-PAN- FTIR studies showed that peak of chitosan at 1164 cm−1 disappeared indicating that this peak may be hindered by cross-linking. When crosslinker was used, FTIR showed appearance of new peak at 1550 cm−1 which can be attributed to cross-linking reaction of chitosan with cross-linking monomer. A new sharp peak appeared at 1627 cm−1 suggested, the stretching of N=C in composite form by the reaction of graft copolymerization. A band at 1564 cm−1 indicated involvement of –NH2 group during grafting.
DSC studies
DSC is the best analytical technique to find the polymer crystallinity, which measures the physical nature of the sample. In this technique a sample is heated or cooled at linear intervals of temperature and the particular temperature and energy accompanied with any one of the range of thermal events is measured. It was observed from DSC plot that chitosan undergoes dehydration at lower temperature as compared to Chito-g-PC. However loss of water from composite was steady and periodic which showed that dehydration pattern was regular in case of DSC of chitosan (Figure S5). DSC of Chito-g-PC showed a weight loss in two distinct stages. The first stage ranges between 205 and 246 °C and showed about 42% loss in weight corresponding to the loss of adsorbed and bound water. This confirms that water does acts as plasticizer at 85 °C in chitosan and its composites. Water forms an intermolecular hydrogen bonding with Chito-g-PC through amine and hydroxyl groups present in them. This helps in molecular rearrangement that eases the chain mobility in Chito-g-PC.
SEM studies
The surface morphology and cross section morphology of Chito-g-polymer composites were characterized by SEM (Figure S6). The SEM micrograph Chito-g-AA-Co-AM, Chito-g-AA, Chito-g-AM, Chito-g-NIPAAM and Chito-g-PAN showed that it was porous structure with many spaces between layers, which may be due to presence of water molecules beneath the region. Interaction between chitosan matrix and monomer may be leading to hydrogen bonding in formation of composites. This develops fine pores and micro voids. The porous structure supports the permeation and interface site of external stimuli with hydrophilic group of Chito-g-polymer composites. Porous morphology of Chito-g-PMMA was slightly different from other Chito-g-polymer composite. It concluded that all composite have porous structure with intermolecular spaces. The size of pore and intermolecular spaces varies depending upon grafted monomer. The pore distribution of composite was comparatively regular. The composites showed high adsorbing surface and high equilibrium adsorption capacity.
XRD studies
Diffraction studies of all synthesized composites revealed the strong interaction between chitosan and the Chito-g-PC as indicated by broad amorphous peak for the entire composites, composites showed 2θ values at 21.44°, 22°, 22.62°, 22.60°, 21.76 and 21.96°. Characteristic crystalline peaks reflection 2θ at 19.34° corresponds to orthorhombic crystal structure of chitosan (Figure S7). The strong intermolecular hydrogen bonding introduced certain regularity in the crystal but composite tends to be more amorphous than pure chitosan. DSC studies revealed that crystalline structure gets converted to amorphous after glass transition point. XRD of pure chitosan and Chito-g-PC showed that crystallinity of chitosan decreases after modification. Similarly shifting in diffraction pattern (~ 19° to ~ 21–22°) of pure chitosan and composites confirmed extensive cross-linking.
Swelling study of polymer composites
The swelling study revealed that the Chito-g-PC were having variable swelling behavior in different pH solutions which contributed to effect on swelling. This indicated that the Chito-g-PC phase transition was well affected pH environment which make it favorable for metal and dyes extraction properties (Fig. 2) [28].
Adsorption studies
Adsorption of MG and MB from aqueous solution was tested in batch experiments. Batch adsorption experiments were carried out to examine effects of adsorbent dosage, initial dye concentration, solution pH, and time on the adsorption of MG and MB on chitosan. Effects of the dye concentrations, pH of the medium, and contact time on the adsorption and capacity were studied and the sorption kinetics was also evaluated [29]. The amount adsorbed was calculated based on the difference of the dye concentrations and the weight of the modified microspheres by the following equation;
where A is the amount of dyes adsorbed onto unit amount of the sorbents (mg/g), Ci is the initial dye concentration (mg/L), Ce is the equilibrium dye concentration (mg/L), V is the volume of dye solution (L), and W is the dry weight of the adsorbents. The adsorption temperature was 25 °C. The initial dyes concentration was 500 mg/L for each MB and MG, respectively. For adsorption experiments, the concentration ranges used were 50–650 and 50–400 mg/L for both MB and MG, respectively (Fig. 3).
The effects of the pH of the sample solutions on the adsorption of dyes evaluated by adjusting the pH by HCl and NaOH. The effect of initial pH on biosorption percentages of dyes were examined over a range of pH values from 2 to 11 for MB and MG (Fig. 4). The dye adsorption for MG were minimum at the initial pH 2. The dyes sorption increased with the increase in the initial pH from pH 2–11 in MB and MG. The solution pH affected the activity of the functional groups (carboxyl) as well as the competition of cationic dyes for the binding sites. At lower pH value, the composite surface became more positively charged, thus increasing the electrostatic repulsive force between the modified Chito-g-PC and cationic dyes. In contrast, as the pH value increased, the composite surface was more negatively changed, which promoted the uptake on the modified Chito-g-PC.
Effects of the MG and MB concentrations and contact time on the adsorption capacity [A]
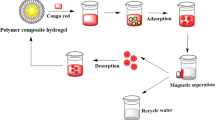
The adsorption of MG and MB from aqueous solution by newly synthesized Chito-g-PC were investigated and shown in Figs. 5 and 6. The percentage adsorptions of dyes on adsorbent were determined by batch methods using UV–Vis spectrophotometer Lambda 650 (PerkinElmer). The effect of experimental parameters, such as pH, treatment time, temperature, adsorbent dose, initial dyes concentration on the removal of dyes were also studied. All Chito-g PC were studied for their cationic dyes properties in terms of Equilibrium Absorption Capacity (A). The initial dye concentration was 500 mg/L for MG and MB. The adsorption mechanisms of MG and MB on surface of composites is described in Fig. 7, as amide from chitosan form strong co-ordinate bond with dyes and were adsorbed on surface chemically.
Thermodynamic parameters
Spontaneity of a process can be determined by thermodynamic parameters such as enthalpy change (∆H°), free energy change (∆G°) and entropy change (∆S°). A spontaneous process will show a decrease in ∆G° and ∆H° values with increasing temperatures [30,31,32,33]. The temperatures used in the thermodynamic study were 300 and 310 K. The thermodynamic parameters were calculated based on the following equations:
Table 1 lists down the values for the thermodynamic parameters. The positive value for the enthalpy change, ΔH° (13.10 kJ/mol), indicated the endothermic nature of the adsorption and explains the increase in MG and MB adsorption efficiency as the temperature increased. The positive value for the entropy change, ΔS° (121.91 J/mol K), indicated that there was an increased disorder at the solid/liquid interface during MG and MB adsorption onto the modified chitosan. The negative value for the free energy change, ΔG°, implies the spontaneity of the adsorption process, which does not require an external energy source for the system.
Adsorption mechanisms of dye on surface of polymer composite
MB and MG poses three binding site to form co-ordinate bond with grafted composite. There was strong co-ordinate interaction between (–CONH2 of grafted composite and –SO3Na end of dye molecule) hence dyes molecule gets absorbed on surface of sorbent by coordinate interaction (Fig. 7).
Reusability of polymer composite
The growing interest in chemical modification of chitosan to improve their solubility and applications requires structural analysis of modified form of chitosan. Degree of deacetylation is one of the most important chemical parameters which affects physicochemical properties of chitosan. Reusability of the modified Chito-g-polymer composite were one of the most important parameters determining the cost effectiveness of the developed method. The recovery of the MB and MG dyes for all the adsorbents were achieved by eluting an appropriate amount of distilled water at various pH. The desorption efficiency of respective dyes were achieved in the said time period for the Chito-g-PC. MB adsorption–desorption shows 98.76% from Chito-g-PC, minimal decrease in adsorption–desorption performance was noted even after eight cycles for MB. Whereas for MG, 97.87% adsorption–desorption from Chito-g-PC was revealed, slightly decreasing elution performance after seven cycles. Therefore, the evaluated data for both dyes implies that adsorption–desorption percentage decreased slightly by the increase in cycles number.
Conclusion
Chitosan based adsorbents have received a lot of attention for adsorption of dyes. Various modifications of this polysaccharide have been investigated to improve the adsorption properties as well as mechanical and physical characteristics of chitosan. Modification of chitosan changes the original properties of this material so that it can be more suitable for adsorption of different types of dyes. In the present investigation novel, environmentally-friendly Chito-g-PC, namely, Chito-g-(AA-co-AM), Chito-g-AM, Chito-g-PAA, Chito-g-NIPAM, Chito-g-PMMA and Chito-g-PAN were prepared by homogeneous grafting by free radical mechanisms. These composites showed higher swelling ratio at pH 5 and Chito-g-(AA-co-AM) showed maximum swelling than other composites at acidic pH. Chito-g-MA composite showed higher sorption capacities than other all composites selectively for MG and MB at acidic pH. Adsorption studies of Chito-g-PMAA for MG and MB showed endothermic behavior. The previous findings indicated that a feasible solution to the drawback of the high degree of swelling of Chito-g-(AA-co-AM) derivatives was devised by other Chito-g-PC. But all those Chito-g-PC can shows metal extraction behavior which could be new horizon in field of biochemistry. In particular, Chito-g-PMAA has all of the sorption characteristics of effective application in large-scale implementations for cationic dye removal from aqueous solutions.
References
Kulal DK, Pansare AV, Tetgure SR, Karve M, Patil VR (2016) Determination of uranium(VI) using Penicillium chrysogenum immobilized on silica gel and spectrophotometer. J Radioanal Nucl Chem 307:1253–1263
Kulal DK, Pansare AV, Shedge AA, Patil VR (2016) Fungal strain of aspergillus oryzae immobilized on silica gel for Au(III) sorption. Eur Chem Bull 5(6):225–231
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Synowiecki J, Al-Khateeb NA (2003) Production, properties, and some new applications of chitin and its derivatives. Crit Rev Food Sci Nutrition 43:145–71
Struszczyk MH (2002) Chitin and chitosan-part I. Properties and production. Polimery 47:316–325
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Nagarkar AA, Kilbinger AFM (2014) End functional ROMP polymers via degradation of a ruthenium Fischer type carbine. Chem Sci 5:4687–4692
Pokhrel D, Viraraghavan T (2004) Treatment of pulp and paper mill wastewater-a review. Sci Total Environ 333:37–58
Thompson G, Swain J, Kay M, Forster CF (2001) The treatment of pulp and paper mill effluent: a review. Bioresour Technol 77:275–286
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026
Zhang S, Dong Y, Yang Z, Yang W, Wu J, Dong C (2016) Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem Eng J 304:325–334
Mahdavinia GR, Pourjavadi A (2004) Modified chitosan Superabsorbent hydrogels from poly(acrylic acid-co-acrylamide) grafted chitosan with salt and pH responsiveness properties. Eur Polym J 40:1399–1407
Babaladimath G, Chapi S (2018) Microwave-assisted synthesis, characterization of electrical conducting and electrochemical xanthan gum-graft-polyaniline. J Mater Sci: Mater Electron 29:11159–11166
Chapi S (2020) Structural and electrochemical properties of polymer blend based ZnO nanocomposite solid polymer electrolytes by spin-coating method. J Nano- Electron Phys 12(2):02043(1)-02043(5)
Tan EKW, Shrestha PK, Pansare AV, Chakrabarti S, Li S, Chu D, Lowe CR, Nagarkar AA (2019) Density modulation of embedded nanoparticles via spatial, temporal, and chemical control elements. Adv Mater 31:1901802
Pansare AV, Khairkar SR, Shedge AA, Chhatre SY, Patil VR, Nagarkar AA (2018) In Situ nanoparticle embedding for authentication of epoxy composites. Adv Mater 30:1801523
Pansare AV, Chhatre SY, Khairkar SR, Bell JG, Barbezat M, Chakrabarti S, Nagarkar AA (2020) “Shape-coding”: morphology-based information system for polymers and composites. ACS Appl Mater Interfaces 12(24):27555–32756
Akkaya R, Ulusoy U (2008) Adsorptive features of chitosan entrapped in polyacrylamide hydrogel for Pb2+, UO22+, and Th4+. J Hazard Mater 151:380–388
Galhoum AA, Mafhouz MG, Gomaa NA, Abdel-Rehem SS, Atia AA, Vincent T, Guibal E (2015) Cysteine-functionalized chitosan magnetic nano-based particles for the recovery of uranium(vi): uptake kinetics and sorption isotherms. Sep Sci Technol 50:2776–2789
Pansare AV, Shedge AA, Chhatre SY, Das D, Murkute P, Pansare SV, Nagarkar AA, Patil VR, Chakrabarti S (2019) AgQDs employing black box synthetic strategy: photocatalytic andbiological behavior. J Lumin 212:133–140134
Pansare AV, Kulal DK, Shedge AA, Patil VR (2016) hsDNA groove binding, photocatalytic activity, and in vitro breast and colon cancer cell reducing function of greener SeNPs. Dalton Trans 45:12144–12155
Chapi S (2020) Optical, electrical and electrochemical properties of PCL5/ITOtransparent conductivefilms deposited by spin-coating-materials forsingle-layer devices. J Sci: Adv Mater Dev 5:322–329
Chapi S (2021) Influence of Co2+ on the structure, conductivity, and electrochemical stability of poly(ethylene oxide)-based solid polymer electrolytes: energy storage devices. J Electron Mater 50(3):1558–1571
Mahdavinia GR, Pourjavadi A (2004) Modified chitosan Superabsorbent hydrogels from poly(acrylic acid-co-acrylamide) grafted chitosan with salt- and pH-responsiveness properties. Eur Polym J 40:1399–1407
Salam OEA, Reiad NA, ElShafei MM (2011) A study of the removal characteristics of heavy metalsfrom wastewater by low-cost adsorbents. J Adv Res 2(4):297–303
Sadeghi M, Yarahmadi M (2011) Synthesis of a novel pH- and salt-responsive super absorbent hydrogel based on collagen-g-poly(AA-co-IA). Orient J Chem 27:453
Kumar D, Raj V, Verma A, Kumar P, Pandey J (2019) Novel binary grafted chitosan nanocarrier for sustained release of curcumin. Int J Biol Macromol 131:184–191
Crini G, Badot P-M (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Ohto K, Inoue S, Eguchi N, Shinohara T, Inoue K (2002) Adsorption behavior of lead ion on calix [4] arenetetracarboxylic acid impregnated resin. Sep Sci Technol 37(8):1943–1958
Pansare AV, Kulal DK, Shedge AA, Patil VR (2016) Green synthesis of anticancerous honeycomb PtNPs clusters: theiralteration effect on BSA and HsDNA usingfluorescence probe. J Photoch Photobiol B 162:473–485
Saha TK, Bhoumik NC, Karmaker S, Ahmed MG, Ichikawa H, Fukumori Y (2010) Adsorption of methyl orange onto chitosan from aqueous solution. J Water Resource Protection 2:898–906
Pansare AV, Shedge AA, Patil VR (2018) Discrete SeNPs-macromolecule binding manipulated by hydrophilic interaction. Int J Biol Macromol 107:1982–1987
Shedge AA, Pansare SV, Khairkar SR, Chhatre SY, Chakrabarti S, Nagarkar AA, Pansare AV, Patil VR (2020) Nanocomposite of functional silver metal containing curcumin biomolecule model systems: protein BSA bioavailability. J. Inorg. Biochem 212:111210
Acknowledgements
A.V.P. acknowledges Composites Group, Mechanical Systems Engineering, Swiss Federal Laboratories for Materials Science and Technology-Empa, 8600 Dübendorf, Switzerland for funding. All authors acknowledges Mr. Chandan Warkar, Promas Pharma Ltd., Mumbai, India, for XRD Analysis facility.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL.
Author information
Authors and Affiliations
Contributions
SRK and SVP authors contributed equally to this work.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khairkar, S.R., Pansare, S.V., Shedge, A.A. et al. Biological macromolecule chitosan grafted co-polymeric composite: bio-adsorption probe on cationic dyes. Polym. Bull. 79, 9441–9455 (2022). https://doi.org/10.1007/s00289-021-03954-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03954-w