Abstract
In our study, a Zn(II) ion-imprinted polymer (ZnIP) and non-imprinted polymer (NIP) were synthesized via free-radical polymerization. 1-Vinylimidazole, ethylene glycol dimethacrylate, 2-hydroxyethylmethacrylate and 2,2′-azobisisobutyronitrile were used as functional, cross-linking monomers and free-radical initiator, respectively. The obtained polymer was characterized by various analytical methods (Fourier Transform Infrared Spectroscopy, Transmission Electron Microscopy, Wavelength Dispersive X-ray Fluorescence, UV VIS, thermal analysis). The sorption properties of ZnIP and NIP were evaluated after removal of Zn(II) ions from the polymer network. The optimum pH for adsorption was 7.0. The maximum adsorption capacity at the pH was 5.2 and 0.22 mg/g for ZnIP and NIP, respectively. To determine the selectivity, the polymer was equilibrated with the binary mixture of Zn(II) ions and the interfering ions [Cu(II), Ni(II) or Co(II)]. The relative selectivity of ZnIP was 22.57, 5.44 and 46.17 for Cu(II), Ni(II) and Co(II) ions, respectively. The proposed ZnIP sorbent was applied to determine the zinc ions in urine samples by Wavelength Dispersive X-ray Fluorescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc plays an important role in human organism. It is a component of over 300 enzymes. The most important of these include (i) DNA and RNA polymerases, which are involved in replication and transcription of genetic material, (ii) superoxide dismutase constituting the first line of defense cells against reactive oxygen species (ROS) and (iii) carbonic anhydrase—conditioning enzyme functions of erythrocytes, the renal tubules, gastric parietal cells and muscle tissue [1, 2]. Moreover, zinc is involved in the synthesis and the breakdown of carbohydrates, lipids, proteins and nucleic acids, and in the metabolism of other trace elements [3]. Both its excess and deficiency can cause a damage to human body systems. Zinc deficiency can lead to symptoms such as growth retardation, loss of appetite and impaired immune function. In more severe cases, it causes hair loss, diarrhea, delayed sexual maturation, impotence, hypogonadism in males, and eye and skin lesions [4,5,6,7].
Dietary allowances (RDAs) for zinc for an adult male are 11 mg and female are 8 mg [4].
Due to the popularity of supplementation of various essential elements, the risk of overdosing zinc rises. Zinc excessive intake may result in a number of adverse health effects including vomiting, fever, nausea, stomach cramps and diarrhea [8]. It is worth mentioning that it may also lead to copper deficiency which is the major consequence of the chronic ingestion of zinc [9]. Additionally, studies in rats revealed that a high-zinc diet induces hypocalcaemia and bone resorption [10]. As a result, it is really important to control the level of Zn in body fluids.
One of the spectroscopic methods that enables evaluation of the elemental composition of various samples is wavelength dispersive X-ray fluorescence (WD-XRF). It has several advantages: short time of analysis; wide dynamic concentration range; solid, powdered and liquid samples can be analyzed. WD-XRF method can be used to quantify zinc in body fluids samples. However, due to the low level of the element, the enrichment of zinc is necessary prior to the analysis. The concentration of Zn in urine of a healthy person lays in the range 0.235–1.976 µg/L [11]. Ion-imprinted polymers can be applied in the urine sample preparation process.
Ion-imprinted polymers are materials which are synthesized in the presence of metal ions. After removal of the template ions from the obtained polymer particles, specific cavities are formed. They are complementary to the template ion used in the synthesis process. The first metal-imprinted polymer was synthesized in 1976 by Nishide et al. [12, 13]. Recently, many researchers developed new ion-imprinted polymers to selectively and effectively adsorb ions from a complex matrix. An example of such materials is zinc(II)-imprinted polymers [14,15,16,17,18,19,20,21,22,23].
Some authors report on preparing zinc(II) ion-imprinted membranes [15, 16]. Kumar et al. developed molecularly imprinted polymer-modified electrochemical sensor for simultaneous determination of copper and zinc [17]. Implementation of ZnIP as a sorbent for solid phase extraction enabled determination of zinc in different matrices including water samples [18,19,20,21], wastewater [22] and food samples [18,19,20,21,22]. Biosorption of zinc(II) ion from water using ion-imprinted interpenetrating polymer network was investigated by Girija and Beena [23].
The aim of this work was the synthesis of a zinc(II) ion-imprinted polymer which is hydrophilic and can be applied to detect zinc in urine samples by WD-XRF.
The WD-XRF method was used for the evaluation of the ion-imprinted polymer properties, such as selectivity and adsorption properties. In this study, a zinc(II)-imprinted polymer was synthesized via a precipitation polymerization. We followed the procedure given by Queila dos Santos et al. [21]. We made some modifications of the synthesis. First, relying on data available in publications we prepared the pre-polymerization compound containing zinc (II) ion and 1-vinylimidazole [24]. The applied molar ratio of 1-vinylimidazole to zinc (II) ions was 4:1. Another modification was based on polymerization in the presence of ethylene glycol dimethacrylate (EDMA) and 2-hydroxyethylmethacrylate (HEMA). The aim of HEMA addition was to increase the surface hydrophilicity of the ZnIP particles [25,26,27]. AIBN was the initiator of the polymerization process.
The properties of the obtained polymer such as characteristics of the structure, selectivity and sorption were evaluated. The polymer was applied to determine the zinc ions in urine samples by WD-XRF.
Materials and methods
Apparatus
The thermogravimetric (TG) and differential thermal analysis (DTA) were done on TGA Q50 (TA instruments) under nitrogen atmosphere. The temperature range was from the room temperature to 700 °C and heating rate was set as 10 °C/min. Infrared spectroscopy (IR) measurements were done using a Perkin Elmer Spectrum 1000 spectrometer. The transmission technique was applied with the use of KBr tablets. The spectra were obtained within the range of 4000–400 cm−1, with 2 cm−1 resolution and 30 scans.
The UV–VIS spectrophotometer (Shimadzu, UV-1800) was used for the surface area determinations. The particles of the obtained polymers were observed with the use of transmission electron microscopy (TEM) (JEM 1400; Jeol Co., Japan). For each test, a drop of ethanol suspension of the investigated powdered polymer was placed on a Cu mesh covered with a formvar film and then allowed to dry in air. The measurements were performed under the accelerating voltage of 80 kV.
To measure the element’s content, WD-XRF spectrometer was used (Thermo, ARL Advant’x series sequential XRF Intelli PowerTM). It was equipped with: the Rh-anode X-ray tube of power 3.6 kW, seven crystals (LiF 200, LiF 220, Ge 111, PET, AX 03, AX 09, AX 16c), two detectors (Flow Proportional Counter and Scintillation Counter) and four collimators (0.15; 0.25; 0.6 and 2.6 mm).
The operating parameters of WD-XRF spectrometer are summarized in Table 1.
Materials
1-Vinylimidazole (1-VI) (>99%), the monomer, ethylene glycol dimethacrylate (EDMA) (98%) and 2-hydroxyethyl methacrylate (HEMA) (>99%), the cross-linking agent, were supplied from Sigma Aldrich (Sigma Aldrich, Germany). 2,2′-Azobisisobutyronitrile (AIBN, Aldrich) was used as an initiator for polymerization and methanol (>99.9%) (Sigma Aldrich, Germany) as the porogenic solvent.
Standard solutions of Zn(II), Cu(II), Ni(II) and Co(II) of concentration 1000 mg/L were purchased from Merck (Darmstadt, Germany). Working solutions were prepared by dilution of stock solutions with deionized water. Concentrated hydrochloric acid (pure for analysis) purchased from Merck (Darmstadt, Germany) was used to prepare 17% HCl (eluent). The Britton–Robinson buffers of certain pH were prepared according to the procedure given in the literature. First, the acidic mixture (0.04 mol phosphoric acid, 0.04 mol boric acid and 0.04 mol acetic acid/L) and sodium hydroxide solution (0.2 mol/L) were prepared [28].Then, the reagents were mixed in appropriate ratios to obtain a buffer of a definite pH. All reagents were of pure analytical grade, POCh (Gliwice, Poland).
Polymer synthesis
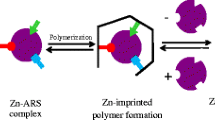
The ZnIP polymer was synthesized via free-radical polymerization (Fig. 1). To form the pre-polymerization complex, ZnSO4·7H2O (1 mmol) was mixed with 1-vinylimidazole (4 mmol) in methanol (20 ml) and the mixture was shaken for 1 h. This solution was then mixed with EDMA (15 mmol), HEMA (5 mmol) and AIBN (25 mg). Oxygen must have been removed from the solution to prevent any unwanted reactions. It was done by bubbling of argon through the mixture for 15 min. The vessel with the mixture was placed in an oil bath and the process was performed 60 °C for 24 h.
After completion of polymerization, the solid polymer was rinsed with 400 ml methanol followed by 100 ml deionized water, crushed, ground and sieved using a sieve at a mesh width of 0.120 mm. To remove the zinc(II) ions from the polymer matrix, the particles were treated with 17% hydrochloric acid. This process was controlled by the WD XRF analysis of the polymer particles and lasted until no Zn could have been detected in the material. The excess of hydrochloric acid was washed by deionized water. Finally, the particles were dried in a vacuum oven at 60 °C.
Simultaneously with ZnIP, non-imprinted polymer (NIP) was prepared. The conditions of the polymerization were similar except for adding the Zn(II) ions.
Procedure
The Zn(II), Cu(II), Co(II) and Ni(II) ions content determination by WD-XRF spectroscopy
The WD-XRF method was used to evaluate the concentration values of Zn(II), Cu(II), Co(II) and Ni(II) in the samples according to our previously developed methodology [29].
Effect of pH
100 mg of ZnIP (or NIP) was placed in a 100 ml-glass bottle followed by addition of an appropriate buffer solution containing zinc (II) ions. The final concentration of Zn(II) was 35 mg/L and the volume of the solution was 20 ml. A set of five test solutions was prepared. The pH value of the solutions varied in the following range 3.00–7.96. The mixtures were shaken at room temperature (20 °C) for 5 h. Subsequently, the polymers were filtered through a membrane filter (Sumplep LCR 25-LG, Nippon Millipore Ltd., Japan), washed with a buffer solution of appropriate pH and dried in a vacuum oven for 12 h.
Static adsorption capacity and adsorption isotherm
To evaluate static absorption capacity, a range of 40 ml solutions was prepared. The concentration range of Zn2+ was as follows: 10–40 mg/L. The amount of 100 mg of ZnIP (or NIP) particles was added into the solution. After adjusting the pH of the mixtures to 7.00, they were shaken at room temperature (20 °C) for 5 h. Subsequently, the polymers were filtered through a membrane filter (Sumplep LCR 25-LG, Nippon Millipore Ltd., Japan), washed with a buffer solution of appropriate pH and dried in a vacuum oven for 12 h.
Adsorption kinetics
A time required for a complete adsorption of Zn2+ on the polymer particles was evaluated. Different sets of ZnIP and NIP samples were prepared. 100 mg of the polymer was immersed in a solution (20 ml) containing 40 mg/L of Zn(II). At certain intervals of time the polymers were filtered off through a membrane filter (Sumplep LCR 25-LG, Nippon Millipore Ltd., Japan), washed with the appropriate buffer solution and dried in a vacuum oven for 12 h. The content of Zn(II) was measured by the WD-XRF method.
Selectivity studies
The selectivity of the synthesized ZnIP was investigated. Competitive adsorption studies of Cu(II)/Zn(II), Co(II)/Zn(II) and Ni(II)/Zn(II) were performed from binary solution of the zinc ions and the competitive ions. To determine the selectivity, 100 mg of the ZnIP (orNIP) was equilibrated with a solution (40 ml) containing zinc (II) and the interfering ion (Cu2+, Co2+ or Ni2+). The concentration of the ions was 40 mg/L. After adjusting the pH to 7.00, the mixtures were shaken at room temperature (20 °C) for 5 h. Subsequently, the polymers were filtered through a membrane filter (Sumplep LCR 25-LG, Nippon Millipore Ltd., Japan), washed with a buffer solution of appropriate pH and dried in a vacuum oven for 12 h.
The distribution ratio (K d), selectivity factor of Zn(II) with respect to Cu(II), Co(II) and Ni(II) (k) and relative selectivity factor (k′) were calculated using the Eqs. 1–3 [30, 31]
where C i is the concentration of Zn(II) in the polymer (mg/g), C e is the equilibrium concentration of Zn (II) (mg/L), K d(Zn) and K d(M) is the distribution ratios of Zn(II) and Cu(II), Co(II) or Ni(II), k i, k n are the selectivity factors of ZnIP and NIP, respectively, and k′ is the relative selectivity factor.
Surface area measurement
Methylene blue adsorption method was applied to measure the surface area of ZnIP and NIP. This method is based on the property of methylene blue to adsorb on solid sorbents as a monolayer [32]. The procedure includes a preparation of a standard stock solution of methylene blue (0.0178 g/L). Then, to create a calibration curve a set of working standards is analyzed by UV–VIS spectrophotometry at λ = 600 nm. The procedure of surface area determination includes equilibration of 0.1 g of ZnIP (or NIP) with 25 ml of methylene blue stock solution. The treatment lasts until the absorbance measured remains the same. The amount of the adsorbed methylene blue is evaluated on the basis of its concentration before and after adsorption.
Application of the zinc ion-imprinted polymer
100 mg of the ZnIP particles was weighed into a glass bottle. Then, 20 ml of the certified urine sample was added to the bottle. After adjusting the pH value to 7.0, the mixture was shaken for 2 h and then the polymer particles were dried overnight (temperature 60 °C). A mortar and pestle made of agate was used to homogenize the samples. Then, accurately weighed powdered samples (50.00 mg) were pressed with the hydraulic press machine (pressure 10 t, time 20 s) to obtain pellets of 13 mm diameter. 150.00 mg of boric acid per each sample was used as backing. The polymer pellets were measured by the WD-XRF method [29].
Results and discussion
Characterization studies
FT-IR spectra
ZnIP was synthesized according to the scheme presented in Fig. 1.
To characterize the synthesized polymer, IR spectroscopy was performed to determine the molecular characteristics of the EDMA/HEMA/1-vinylimidazole matrix.
The IR spectra of unleached zinc(II) imprinted polymer (ZnIP) and non-imprinted polymer (NIP) materials were recorded (Fig. 2).
FT-IR analysis confirmed that the ZnIP synthesis was successful. This conclusion is based on the observation that the majority of the spectra bands for the ZnIP and NIP polymers are similar.
Absorption peaks at 2990 and 2952 cm−1 correspond to the stretching frequencies of methyl(ene) groups. Absorption due to carbonyl vibration of the EDMA (1728 cm−1) was observed. C–O stretching of the ester is represented by broad 1265 and 1159 cm−1 bands, whereas the weak absorptions at 1638 and 1456 cm−1 are assigned to the C=C and C=N structure of the aromatic imidazole ring. The absorption at 962 and 765 cm−1 is assigned to the C–H (ring) bending out-of-plane [33, 34]. The peak at 1380 cm−1 is attributed to C=N stretching modes from the ring [21]. The strong and broad absorption bands around 3700–3100 cm−1 correspond to water O–H stretching.
The lack of IR bands in the range of 1500–1600 cm−1 proves complete polymerization process [35,36,37].
WD-XRF spectra of ZnIP and NIP after synthesis
WD-XRF method enables detection and quantification of elements in solid samples including polymers. In ion imprinted polymers experiments it is very useful in confirmation that the metal ions were incorporated in the polymer network in the synthesis process. In our study, we recorded the WD-XRF spectra of ZnIP and NIP (Fig. 3).
Analysis of the spectrum shows that a signal in the 2 Theta angle range of 59.6–69.6 appears only for ZnIP. Quantitative analysis of Zn(II) in the polymer can be used to evaluate the concentration of zinc. The resulting concentration of zinc was 1.65% and below the detection limit in the ZnIP and NIP, respectively.
The theoretical concentration of zinc in the final polymer should be 1.85%. Relying on these data, the yield of the reaction can be estimated—89.2%.
Surface area measurement
The surface area of ZnIP and NIP was calculated using the Eq. 4 [32].
where A s is the imprinted polymer surface area in m2 g−1, G is the amount of methylene blue adsorbed (g), N AV is the Avogadro’s number (6.02 × 1023 mol−1), Ø is the methylene blue molecular cross-section (197.2 Á2), M W is the molecular weight of methylene blue (373.9 g/mol), and M is the mass of adsorbent (g).
Surface area measurements done by methylene blue adsorption experiments revealed a higher surface area for leached ZnIP than for NIP (15.92 and 12.41 m2/g, respectively). The surface area of ZnIP is higher than that of NIP because leaching the Zn2+ ions from the polymer matrix leads to a formation of specific cavities in the polymer network.
TEM results
The crystal’s morphology and dimensions were evaluated on the basis of TEM images (Fig. 4a, b). NIP particles had the tendency to form compact agglomerates. The ZnIP samples had particles of elongated, rod-like shape. The mean length and width of ZnIP particles did not exceed 400 and 80 nm, respectively.
Thermal analysis of the polymers
Thermal stability of the leached and unleached ZnIP particles was investigated by TG and DTA analysis. The results in Fig. 5a, b show that the TGA/DTA curves of leached and unleached ZnIP particles are similar. This implies that the elution of zinc ions did not significantly affect the thermal properties of the ZnIP particles.
The peak at 405 °C present on DTA diagram for both unleached and leached ZnIP particles originates from the major exothermic decomposition of the polymer.
There are two steps of thermal decomposition of the ZnIP particles. The first step is observed below the temperature of 200 °C. It corresponds to a slight weight loss of 1.3% for both leached and unleached ZnIP. The second stage of decomposition was started at 200 °C and continued until 500 °C (leached ZnIP) or 550 °C (unleached ZnIP). The weight loss at this stage is 90.2 and 90.3% for leached and unleached ZnIP particles, respectively.
Due to the fact that the decomposition of unleached ZnIP ends at higher temperature than that of leached ZnIP particles, it can be assumed that it is more stable and has more robust composition and structure.
Sorption studies
Effect of pH
pH of the solution is one of the most important parameters affecting adsorption of the metal ions on the polymer particles. Thus, using a batch procedure we investigated the effect of pH variation on the adsorption of Zn(II) in the following pH range 3.00–7.96.
As can be observed in Fig. 6, the adsorption of Zn(II) ions on the polymer particles is not very high below pH 4. It is probably due to the protonation of the polymers [30, 38, 39]. With the increasing pH, the adsorption capacity increases reaching its maximum in the following pH range 5.0–8.0.
7.0 is the pH considered to be optimum and was used in further experiments.
Static adsorption capacity
Adsorption capacity (Q) is one of the most important feature of ion-imprinted polymers. It is expressed as the entire amount of the adsorbed ions per gram of the polymer sample [18]. In our experiments, we determined the static adsorption capacity, which is defined as the highest concentration of the metal ions that is adsorbed by the polymer at equilibrium [40].
Figure 7 shows the relationship between sorption capacity and the initial concentration of Zn(II) ions in the aqueous solution.
As shown in Fig. 7, the quantity of zinc ions adsorbed per unit mass of the polymer particles increased up to saturation with the initial concentration of Zn2+. The maximum static adsorption capacity for ZnIP and NIP was 5.2 and 0.22 mg/g, respectively. A comparison of the adsorption capacity of the imprinted polymer and non-imprinted polymer is very important, because it allows confirmation of imprinting efficiency (the presence of the polymer structure-specific cavities that are able do adsorb only the zinc ions). The zinc ion-imprinted polymers described in prior publications had the adsorption capacity in the range of 2.73 and 68.6 mg/g [14, 18,19,20, 22, 23]. It is worth emphasizing that not all the authors did determine the adsorption capacity of the non-imprinted polymer and others did not determine the adsorption capacity at all.
Our ZnIP can adsorb zinc ions 24 times more effectively than the NIP. If we compare the values with those available in the literature (1.2–8.6), it is the highest value [19, 22, 23].
Isotherm study
The adsorption properties of ZnIP particles were determined in batch experiments. The Langmuir adsorption isotherm was applied in interpreting Zn2+ adsorption capacity of the imprinted polymer. There are several assumptions of the Langmuir model: (i) the binding sites are homogenous; (ii) the molecules are adsorbed at a fixed number of well-defined sites, each of which can hold only one molecule; (iii) the sites are assumed to be energetically equivalent and distant to each other so that there are no interactions between molecules adsorbed on adjacent sites [41, 42].
The Langmuir adsorption isotherm can be represented by the Eq. 5 [43,44,45].
where C e is the equilibrium concentration of metal ions (mg/L), Q is the amount of metal ions adsorbed (mg/g), Q m is the maximum adsorption capacity of metal ions (mg/g), and b is the Langmuir adsorption equilibrium constant (L/mg) [46, 47]. If a graph showing the relationship between C e/Q and C e is drawn, we obtain a linear function. The slope of the graph is 1/C m and the intercept is 1/(Q m b).
The linear plot of Langmuir Isotherm is shown in Fig. 8.
A high value of R 2 (R 2 = 0.992) of the Langmuir model plot indicates that the adsorption was monolayer and adsorption of each zinc(II) ion had similar activation energy. The maximum adsorption capacity Q m was 5.29 mg/g.
Adsorption kinetics
To determine the optimum contact time, metal sorption capacity was determined as a function of time (Fig. 9). 100 mg of the ZnIP particles after leaching was equilibrated with 20 ml of a Zn(II) solution of concentration 40 mg/L. After 5, 7.5, 10, 20 and 30 min, the polymer was filtered off and the Zn(II) content was measured in the polymer by the WD XRF method. The results show that after 10 min the Zn(II) ions are effectively bound to the polymer.
The sorption kinetics data for ZnIP were also analyzed using pseudo-second-order equation (Eq. 6).
where k 2 [g/(mg min)] is the second-order Lagergren constant of adsorption, and q t (mg/g) and q e (mg/g) are the quantities of metal ions adsorbed at time t (min) and at equilibrium, respectively.
In this model, the rate-limiting step is the surface and sorption that involves chemisorption, where the removal from a solution is due to physicochemical interactions between the two phases [48].
The results in Fig. 10 indicate that the parameter Q/t is highly correlated to time (R 2 = 0.9962). This means that our results fit to the pseudo-second-order Lagergren kinetic model. The value of k 2 calculated from the slope is 0.202 g mg−1 min−1.
Selectivity studies
It can be assumed that metal ions of the same charge and similar iconic radii as Zn2+ can influence the adsorption process of zinc ions. Thus, competitive sorption of Zn2+/Cu2+, Zn2+/Ni2+ and Zn2+/Co2+ from their binary mixtures was investigated in batch experiments. The selected interfering ions possess a similar charge and ionic radii (Zn(II) = 74 pm, Cu(II) = 71 pm, Ni(II) = 69 pm and Co(II) = 72 pm) [49]. The results in Table 2 indicate that depending on the interfering ion referring to Zn2+ the relative selectivity coefficients were 5.44–46.2 times greater than those of the non-imprinted polymers. It proves that the obtained Zn(II) ion-imprinted polymer is able to adsorb Zn2+ ions from the samples of a complex matrix containing metal ions of similar charge and radii.
Determination of Zn2+ ion in urine samples
The ion-imprinted polymer was applied to enrich the zinc ions from the urine samples. WD-XRF method is not used for routine direct zinc determination in urine samples due to the low concentration of zinc ions in the samples. Selective adsorption of zinc on the ZnIP particles enables detection and quantitative analysis of zinc in urine by WD-XRF. The method of determination was tested on 5 urine samples including one reference material (Seronorm™ Trace Elements Urine) (Table 3). The percentage recovery of all the results is in the following range 87–97%.
Table 4 shows a comparison of the values of the adsorption capacity and relative selectivity coefficients of the new ZnIP and polymers reported in the literature.
It should be noted that the selectivity towards the cobalt(II) ions is the best among the polymers reported in the literature. Although the adsorption capacity of the polymer characterized in this work is lower than some other zinc(II) ion-imprinted polymers, its properties seem to be a compromise between sorption capacity and selectivity.
Conclusions
In this work, a Zn(II) ion-imprinted polymer was prepared and characterized. Prior to the precipitation, polymerization the complex of zinc(II) and 1-vinylimidazole was prepared in methanol. The cross-linking monomers and initiator were ethylene glycol dimethacrylate and 2-hydroxyethyl metacrylate and 2,2′-azobisisobuthyronitrile, respectively. The sorbent exhibits relatively high adsorption capacity and good selectivity towards interfering ions such as: Cu2+, Ni2+ and Co2+. Results from the analysis of urine samples have shown that the developed method can be successfully applied for the zinc determination in urine by the WD-XRF method.
References
Coleman JE (1992) Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem 61:897–946
Christianson DW, Fierke CA (1996) Carbonic anhydrase: evolution of the Zinc binding site by nature and by design. Acc Chem Res 29:331–339
Human Vitamin and Mineral Requirements (2001) Report of a joint FAO/WHO expert consultation, Bangkok, Thailand. Food and Agriculture Organization of the United Nations. World Health Organization. Food and Nutrition Division FAO Rome
Institute of Medicine, Food and Nutrition Board (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press, Washington
Maret W, Sandstead HH (2006) Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 20:3–18
Prasad AS (2004) Zinc deficiency: its characterization and treatment. Met Ions Biol Syst 41:103–137
Wang LC, Busbey S (2005) Images in clinical medicine. Acquired acrodermatitis enteropathica. N Engl J Med 352:1121–1127
Elinder CG (1986) Zinc. In: Friberg L, Nordberg GF, Vouk VB (eds) Handbook on the toxicology of metals, 2nd edn. Elsevier Science Publishers, Amsterdam, pp 664–679
Cousins RJ, Hempe JM (1990) Zinc. In: Brown ML (ed) Present knowledge in nutrition, 6th edn. International Life Science Institute - Nutrition Foundation, Washington, DC, pp 251–260
Yamaguchi M, Takahashi K, Okada S (1983) Zinc-induced hypocalcemia and bone resorption in rats. Toxicol Appl Pharmacol 67:224–228
Bamgbose O, Opeolu OB, Bamgbose JT (2007) Levels of Cadmium, Lead and Zinc in Urine of randomly selected smokers and non-smokers residents of Abeokuta City, Nigeria. J Appl Sci Res 2:198–203
Nishide H, Deguchi J, Tsuchida E (1976) Selective adsorption of metal ions on crosslinked poly(vinylpyridine) resin prepared with a metal ions as a template. Chem Lett 5:169–176
Nishide H, Deguchi J, Tsuchida E (1977) Adsorption of metal ions on crosslinked poly(4-vinylpyridine) resins prepared with a metal ion as template. J Polym Sci Polym Chem 15:3023–3029
Kim M, Jiang Y, Kim D (2013) Zn2+-imprinted porous polymer beads: synthesis, structure, and selective adsorption behavior for template ion. React Funct Polym 73:821–827
Araki K, Maruyama T, Kamiya N, Goto M (2005) Metal ion-selective membrane prepared by surface molecular imprinting. J Chromatogr B 818:141–145
Zhai Y, Liu Y, Chang X, Ruan X, Liu J (2008) Metal ion-small molecule complex imprinted polymer membranes: preparation and separation characteristics. React Funct Polym 68:284–291
Kumar D, Madhuri R, Prasad TM, Sinha P, Bali PB (2011) Molecularly imprinted polymer-modified electrochemical sensor for simultaneous determination of copper and zinc. Adv Mat Lett 2:294–297
Shakerian F, Dadfarnia S, Shabani AMH (2012) Synthesis and application of nano-pore size ion imprinted polymer for solid phase extraction and determination of zinc in different matrices. Food Chem 134:488–493
Behbahani M, Salarian M, Bagheri A, Tabani H, Omidi F, Fakhari A (2014) Synthesis, characterization and analytical application of Zn(II)-imprinted polymer as an efficient solid-phase extraction technique for trace determination of zinc ions in food samples. J Food Comp Anal 34:81–89
Shamsipur M, Rajabi HR, Pourmortazavi SM, Roushani M (2014) Ion imprinted polymeric nanoparticles for selective separation and sensitive determination of zinc ions in different matrices. Spectrochim Acta A Mol Biomol Spectrosc 117:24–33
dos Santos QO, Bezerra MA, Lima GF, Diniz KM, Segatelli MG, Germiniano TO, Silva Santos V, Tarley CRT (2014) Synthesis, characterization and application of ion imprinted poly(vinylimidaole) for zinc extraction/preconcentration with FAAS determination. Quim Nova 37:63–68
Zhao J, Han B, Zhang Y, Wang D (2007) Synthesis of Zn(II) ion-imprinted solid-phase extraction material and its analytical application. Anal Chim Acta 603:87–92
Girija P, Beena M (2013) Biosorption of toxic Zn(II) ion from water using ion imprinted interpenetrating polymer networks. J Chem Eng 7:508–517
Goodgame DML, Goodgame M, Rayner-Canham GW (1969) Spectroscopic studies of substituted imidazole complexes. II. N-methylimidazole complexes of divalent cobalt, nickel, copper and zinc. Inorg Chim Acta 3:406–410
Oral E, Peppas NA (2006) Hydrophilic molecularly imprinted poly(hydroxyethyl-methacrylate) polymers. J Biomed Mater Res A 78:205–210
Yavuz H, Say R, Denizlia A (2005) Iron removal from human plasma based on molecular recognition using imprinted beads. Mater Sci Eng C 25:521–528
Branger C, Meouche W, Margaillan A (2013) Recent advances on ion-imprinted polymers. React Funct Polym 73:859–875
Meites L (1963) Handbook of analytical chemistry. McGraw-Hill Book Company, New York
Kuras M, Więckowska E (2015) Synthesis and characterization of a new copper(II) ion-imprinted polymer. Polym Bull 72:3227–3240
Shamsipur M, Fasihi J, Khanchi A, Hassani R (2007) A stoichiometric imprinted chelating resin for selective recognition of copper(II) ions in aqueous media. Anal Chim Acta 599:294–301
Nacano LR, Segatelli MG, Tarley CRT (2010) Selective sorbent enrichment of nickel ions from aqueous solutions using a hierarchically hybrid organic-inorganic polymer based on double imprinting concept. J Braz Chem Soc 21:419–430
Kaewprasit C, Hequet E, Abidi N, Gourlot JP (1998) Application of methylene blue adsorption to cotton fiber specific surface area measurement. Part 1 methodology. J Cotton Sci 2:164–173
Segatelli MG, Santos VS, Presotto ABT, Yoshida IVP, Tarley CRT (2010) Cadmium ion-selective sorbent preconcentration method using ion imprinted poly (ethylene glycol dimethacrylate-co-vinylimidazole). React Funct Polym 70:325–333
Tarley CRT, Andrade FN, Santana H, Zaia DAM, Beijo LA, Segatelli MG (2012) Ion-imprinted polyvinylimidazole-silica hybrid copolymer for selective extraction of Pb(II): Characterization and metal adsorption kinetic and thermodynamic studies. React Funct Polym 72:83–91
Lai X, Hu Y, Fu Y, Wang L, Xiong J (2012) Synthesis and characterization of Lu(III) ion imprinted polymer. J Inorg Organomet P 22:112–118
Mafu LD, Msagati TAM, Mamba BB (2013) Ion-imprinted polymers for environmental monitoring of inorganic pollutants: synthesis, characterization, and applications. Environ Sci Pollut Res 20:790–802
Firouzzare M, Wang Q (2012) Synthesis and characterization of a high selective mercury(II) imprinted polymer using novel aminothiol monomer. Talanta 101:261–266
Kanazawa R, Mori K, Tokuyama H, Sakohara S (2004) Preparation of thermosensitive microgel adsorbent for quick adsorption of heavy metal ions by temperature change. J Chem Eng Jpn 37:804–807
Zhai Y, Yang D, Chang X, Liu Y, He Q (2008) Selective enrichment of trace copper(II) from biological and natural water samples by SPE using ion-imprinted polymer. J Sep Sci 31:1195–1200
Tsoi YK, Ho YM, Leung KS-Y (2012) Selective recognition of arsenic by tailoring ion-imprinted polymer for ICP-MS quantification. Talanta 89:162–168
Hasan S, Krishnaiah A, Ghosh TK, Viswanath DS, Boddu VM, Smith ED (2003) Adsorption of chromium (VI) on chitosan coated perlite. Sep Sci Technol 38:3775–3793
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Hoai NT, Yoo DK, Kim D (2010) Batch and column separation characteristics of copper-imprinted porous polymer micro-beads synthesized by a direct imprinting method. J Hazard Mat 173:462–467
Hoai NT, Kim D (2009) Synthesis, structure, and selective separation behavior of copper-imprinted microporous polymethacrylate beads. AIChE 55:3248–3254
Chen AH, Yang CY, Chen CY, Chen CY, Chen CW (2009) The chemically crosslinked metal-complexed chitosans for comparative adsorptions of Cu(II), Zn(II), Ni(II) and Pb(II) ions in aqueous medium. J Hazard Mat 163:1068–1075
Niu C, Wu W, Wang Z, Li S, Wang J (2007) Adsorption of heavy metal ions from aqueous solution by crosslinked carboxymethyl konjac glucomannan. J Hazard Mater 141:209–214
Ng JCY, Cheung WH, McKay G (2003) Equilibrium studies for the sorption of lead from effluents using chitosan. Chemosphere 52:1021–1030
Wang H, Zhou A, Peng F, Yu H, Yang J (2007) Mechanism study on adsorption of acidified multi-walled carbon nanotubes to Pb(II). J Colloid Interface Sci 316:277–283
Dam AH, Kim D (2008) Metal ion-imprinted polymer microspheres derived from copper methacrylate for selective separation of heavy metal ions. J Appl Polym Sci 108:14–24
Acknowledgements
This work was supported by the Medical University of Warsaw within the student Grant FW23/NM1/14. The TEM studies were performed in the Laboratory of Electron Microscopy, Nencki Institute of Experimental Biology, Warsaw, Poland. We used equipment installed within the project sponsored by the EU Structural Funds: Centre of Advanced Technology BIM—Equipment purchased for the Laboratory of Biological and Medical Imaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kuras, M.J., Perz, K. & Kołodziejski, W.L. Synthesis, characterization and application of a novel zinc(II) ion-imprinted polymer. Polym. Bull. 74, 5029–5048 (2017). https://doi.org/10.1007/s00289-017-1987-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-1987-1