Abstract
In this study, a new copper(II) ion-imprinted polymer (Cu(II)-IIP) was synthesized by the precipitation method. Itaconic acid, ethylene glycol dimethacrylate and 2,2′-azobisisobutyronitrile were used as functional, cross-linking monomer and a free-radical initiator, respectively. This polymer has been characterized on the basis of Fourier transform infrared spectroscopy and surface area measurements. The imprinted Cu(II) ions were completely removed from the polymer by leaching with the mixture of 0.1 M EDTA and 1 M HCl. The optimum pH for the adsorption of Cu(II) on to the polymer was 6. The selective performance of the polymer was compared to non-imprinted polymer (NIP) for the binary mixture Cu2+/Ni2+ and Cu2+/Zn2+. The relative selectivity of Cu(II)-IIP was 12.8 and 32.4 times greater than that of NIP as compared with the Zn2+ and Ni2+ ions, respectively. At optimal pH value, the maximum static adsorption capacity of Cu(II)-IIP and NIP was found to be 14.8 and 4.08 mg/g, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper is a common environmental pollutant, which is encountered with food and drink in urban countries. The World Health Organization (WHO) evaluated regulations concerning the copper(II) content in drinking water. The maximum recommended concentration of Cu(II) in drinking water is 2 mg/L (31 mM) [1]. Copper is an essential trace element and plays an important role in various biological systems. It is a functional component of essential enzymes (eg. cytochrome c oxidase, lysyl oxidase, ferroxidase, tyrosinase, dopamine beta-monooxygenase, d-hexozo oxidoreductase, superoxide dismutase), takes part in cell transpiration, etc. [1–4]. Both, its excess and deficiency can cause damage to human body systems. Copper deficiency can lead to symptoms such as hypochromic anemia, leucopenia and osteoporosis [5]. On the other hand, its excessive intake may result in a number of adverse health effects including liver and kidney damage, immunotoxicity and developmental toxicity [6]. As a result, it is really important to control the concentration of Cu in body fluids as well as environmental samples (sewage sludge, drinking water), which is usually accomplished by atomic absorption spectroscopy, inductively coupled plasma optical emission spectroscopy and inductively coupled plasma mass spectrometry methods. The concentration of Cu in urine and blood of a healthy person lays in the range 13–108 µg/L [7]. The presence of Ni, Zn and Cu at the similar concentration level in those samples can lead to matrix effects, which influence the accuracy of Cu determination by ET AAS [8]. Due to the complexity of the samples’ matrix, often prior to their analysis, it is necessary to separate and enrich the copper ions using the following methods: liquid–liquid extraction [9, 10], co-precipitation [11], solid-phase extraction (SPE) [12, 13] and cloud point extraction [14, 15]. The basic principle of SPE is the transfer of analytes from the aqueous phase to the active sites of the adjacent solid phase, which is stimulated by the selection of appropriate optimal conditions in the system of three major components: water (liquid phase); analyte; and, sorbent [16]. It seems that among the methods of solid-phase extraction those where copper(II) ion-imprinted polymers were used were relatively simple to perform [17].
The first metal-imprinted polymers were synthesized in 1976 by Nishide et al. [18, 19]. They co-polymerized poly(4-vinylpyridine) with 1,4-dibromobutane in the presence of metal ions. Next, they analyzed the adsorption of Cu(II), Zn(II), Co(II), Ni(II), Hg(II) and Cd(II) on acquired resins. It turned out that the resins preferably adsorbed the metal ions, which have been used in the process of synthesis. In the past years, a rapid increase in number of publications concerning metal-imprinted polymers including copper(II)-imprinted polymers, their characteristics and application can be observed [20–41]. Baghel et al. reported a fabrication of an ion-selective electrode using Cu(II) ion-imprinted polymer for sensing Cu(II) ion [33]. Cu(II)-IIP were also used in the development of a fluorescent Cu(II)-IIP-based optosensor [41, 56]. These materials can also be applied as sorbents in SPE, which enable selective determination of copper in various water samples: mineral water, seawater, distilled water, well water, river water, spring water and tap water samples from water installation containing copper elements [21, 23, 25, 26, 31, 32, 37, 40]. Besides water analysis, Cu(II)-IIP was used in a selective preconcentration for a trace Cu(II) determination in biological samples, such as human urine, serum samples [42] and fish samples [32].
The metals analysis performed when determining important parameters of the ion-imprinted polymers was carried out by the following methods: atomic absorption spectrometry [25, 29, 32, 33, 37, 38, 57] and inductively coupled plasma optical emission spectrometry [31, 35, 40, 42].
In our work, we applied wavelength-dispersive X-ray spectroscopy (WD-XRF), which effectively allows for a quantitative determination of an element directly in the ion-imprinted polymer matrix. WD-XRF has been used for the determination of metals in polymers. It is a non-destructive method that enables determination of elements in solid-state samples. This technique has been applied very successfully to industries, to the routine determination in polyethylene and polypropylene samples of the following elements: aluminum, bromine, calcium, chlorine, magnesium, potassium, sodium, titanium and vanadium [43]. The WD-XRF method was also used for the determination of elements in the commercial polymers produced by zirconocene (Al), Ziegler-Natta (Ti and V), Philips (Cr) and metallocene (Zr) technology [44, 45]. Another application of this technique is the determination of metals (Co, Cu, Fe, Ni and Zn) in polybutadiene, polyisoprene and polyester resins [46].
The aim of this work was the synthesis of a copper(II) ion-imprinted polymer, which applied in the solid-phase extraction system, can selectively adsorb copper(II) ions from the samples of a complex matrix, including biological and environmental samples. The analytical method—the wavelength-dispersive X-ray spectrometry was for the first time used for the evaluation of the ion-imprinted polymer properties, such as selectivity, adsorption properties, etc. In this study, a new copper(II)-imprinted polymer was synthesized via a precipitation polymerization. Itaconic acid was used to prepare a pre-polymerization compound with the copper (II) ion. The polymerization was performed in the presence of EDMA and AIBN as a cross-linking monomer and initiator, respectively. The properties of the obtained polymer such as characteristics of the structure, selectivity and sorption were evaluated.
Materials and methods
Apparatus
In this study, the following spectrometers were used: the Fourier Transform Infrared (FT-IR) spectrometer (Perkin Elmer, SPECTRUM 1000), the UV–VIS spectrophotometer (Shimadzu, UV-1800) and wavelength-dispersive X-ray spectrometer (WD-XRF) (Thermo, ARL Advant’x series sequential XRF Intelli PowerTM) equipped with: the Rh-anode X-ray tube of power 3,6 kW, seven crystals (LiF 200, LiF 220, Ge 111, PET, AX 03, AX 09, AX 16c), two detectors (Flow Proportional Counter and Scintillation Counter) and four collimators (0.15; 0.25; 0.6; 2.6 mm).
The operating parameters of the WD-XRF spectrometer are summarized in Table 1.
Materials
Itaconic Acid (IA) (>99 %), the monomer and ethylene glycol dimethacrylate (EDMA) (98 %), the cross-linking agent, were supplied from Sigma Aldrich (Sigma Aldrich, Germany). 2,2′-Azobisisobutyronitrile (AIBN, Aldrich, Germany) was used as an initiator for polymerization and methanol (>99.9 %) (Sigma Aldrich, Germany) was used as the porogenic solvent.
Stock solutions of Cu(II), Ni(II) and Zn(II) of concentration 1000 mg/L were purchased from Merck (Darmstadt, Germany). Working solutions were prepared by diluting the stock solutions with deionized water. The eluent solutions were prepared by dilution of concentrated hydrochloric acid (pure for analysis) and EDTA (<99 %), purchased from Merck (Darmstadt, Germany). The Britton–Robinson buffers were prepared according to the procedure given in the literature [47], by mixing the acidic solution (0.04 mol phosphoric acid, 0.04 mol boric acid and 0.04 mol acetic acid L−1) with 0.2 mol L−1 sodium hydroxide solution in appropriate ratios. All reagents were of pure for analysis grade, POCh (Gliwice, Poland).
Polymer synthesis
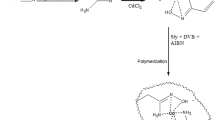
Cu(II)-IIP (Fig. 1) was prepared by thermal polymerization. Copper (II) ion (1 mmol) was mixed with itaconic acid (2 mmol) in methanol (30 mL). This solution was then mixed with ethylene glycol dimethacrylate (20 mmol) and 2,2′-azobisisobutyronitrile (50 mg). To prevent any side reactions, the oxygen of the solution was removed by bubbling of argon through it for 15 min. The polymerization reaction was performed in an oil bath at 60 °C for 24 h. After completion of polymerization, the solid polymer was crushed and ground. To remove the template ion, the particles were treated with the mixture of 2 M HCl and 0.1 M EDTA until no copper could be detected in the polymer material by the WD-XRF method. The excess amount of HCl/EDTA was washed by methanol and deionized water. Finally the particles were dried in a vacuum oven at 60 °C.
Non-imprinted polymer (NIP) was also prepared under similar conditions except for adding the template ion.
Sample preparation
Calibration standards preparation
The WD-XRF method was used to evaluate the concentration values of Cu(II), Ni(II) and Zn(II) in the samples.
First, calibration curves were constructed by measuring a series of ten calibration standard samples containing a known amount of Cu (II), Ni(II) and Zn(II) ions. The procedure of preparing calibration standards was as follows: 2.000 g of microcrystalline cellulose was weighed into glass bottles. To that quantity 50 mL of a standard solution of metal ions (Cu(II), NI(II) and Zn(II)) of known concentration was added. The mixtures were shaken for 2 h and finally the standard samples were dried in a vacuum oven for 72 h (temperature 60 °C). A mortar and pestle made of agate was used to homogenize the samples. Next, accurately weighed powdered standard samples (50.00 mg) were pressed with the hydraulic press machine (pressure 10 t, time 20 s) to obtain pellets of 13 mm diameter. 150.00 mg of boric acid per each sample was used as backing. The backing absorbs stress and shock in the pressing process, and gives a smooth pellet surface. The standard pellets were measured by the WD-XRF method. The calibration curves were prepared in the following concentration range: 0.020–20 mg/g. The linearity of the calibration curves for Cu(II), Ni(II) and Zn(II) was satisfactory—the value of R 2 was 0.9935, 0.9984 and 0.9958, respectively.
The preparation of the polymer samples
All of the analyzed polymer samples were homogenized with a mortar and pestle made of agate. Next, accurately weighed powdered polymer samples (50.00 mg) were pressed with the hydraulic press machine (pressure 10 t, time 20 s) to obtain pellets of 13 mm diameter. 150.00 mg of boric acid per each sample was used as backing. The polymer pellets were measured by the WD-XRF method. The concentration of Cu, Ni and Zn in the polymer samples was determined from the obtained calibration curves.
Procedure
Effect of pH
The samples of the Cu(II)-IIP and NIP (50 mg) were equilibrated with the solution of copper (II) ions and the buffer in 100-mL glass bottles. The total concentration of Cu(II) was 10 mg/L and the volume 40 mL. The set of five test solutions of pH varying from 2.09 to 6.09 was prepared. The solutions were shaken for 5 h at room temperature (20 °C). Then the polymers were filtered off through a membrane filter (Sumplep LCR 25-LG, Nippon Millipore Ltd., Japan), washed with the appropriate buffer solution and dried overnight in a vacuum oven.
Static adsorption capacity
A sample of 100 mg of Cu(II)-IIP (or NIP) particles was added into a 50-mL solution containing Cu2+ in the concentration of 10 ppm, and the solution was sealed in a test bottle (100 mL volume). The pH was adjusted to 6.0 and the mixture was shaken for 5 h at a room temperature (20 °C). Then the polymers were filtered off through a membrane filter (Sumplep LCR 25-LG, Nippon Millipore Ltd., Japan), washed with the appropriate buffer solution and dried overnight in a vacuum oven.
Adsorption kinetics
The adsorption isotherm was evaluated in batch experiments. Seven solutions of Cu(II) over the concentration range of 0.7–10 ppm were maintained at the optimum pH and shaken with 100 mg of Cu(II)-IIP. The mixtures were shaken for 5 h at room temperature (20 °C). Then the polymers were filtered off through a membrane filter (Sumplep LCR 25-LG, Nippon Millipore Ltd., Japan), washed with the appropriate buffer solution and dried overnight in a vacuum oven.
Selectivity studies
The selectivity of the Cu(II)-IIP towards Cu2+ in the presence of competitive ions was investigated. The sorption studies were performed from binary solution of the copper ions and the competitive ions. 100 mg of the polymer (Cu(II)-IIP or NIP) was placed in a glass bottle (250 mL) and mixed with a solution containing Cu2+ (10 mg/L) and Ni2+ (1 or 10 mg/L) or Zn2+ (1 or 10 mg/L). After adjusting pH to 6.0, the solutions were shaken for 5 h at room temperature (20 °C). Then the polymers were filtered off through a membrane filter (Sumplep LCR 25-LG, Nippon Millipore Ltd., Japan), washed with the appropriate buffer solution and dried overnight in a vacuum oven.
The distribution ratio (D), selectivity factor of Cu(II) with respect to Zn(II) and Ni(II) (α) and relative selectivity factor (α r) were calculated using the Eqs. 1–3 [21, 48].
where C i is the concentration of Cu(II) in the polymer (mg/g), C e is the equilibrium concentration of Cu (II) (mg/L), D Cu and D M is the distribution ratios of Cu(II) and Zn(II) or Ni(II), α i, α n is the selectivity factors of Cu(II)-IIP and NIP, respectively, α r is the relative selectivity factor.
Surface area measurement
The surface area of the leached Cu(II)-IIP and NIP was measured by methylene blue adsorption method. Methylene blue is known to be adsorbed as a monolayer only on solid adsorbents [49]. A standard stock solution of methylene blue was prepared (0.0178 g L−1). A set of the working standards was analyzed by UV–VIS spectrophotometry (λ = 600 nm) to draw a calibration curve for methylene blue. To calculate the surface area, 0.1 g of Cu(II)-IIP and NIP was treated with 25 mL of methylene blue solution of concentration 0.0178 g L−1. The treatment lasted until there was no further decrease in absorbance. The amount of methylene blue adsorbed was calculated based on the difference between the initial and equilibrium concentration, which were measured spectrophotometrically.
Results and discussion
Characterization of polymer
Figure 1 represents the scheme of the reactions that led to prepare Cu(II)-IIP.
The IR spectra of unleached copper (II)-imprinted polymer (Cu(II)-IIP) and non-imprinted polymer (NIP) materials were recorded using KBr pellet method (Fig. 2).
The majority of the FT-IR spectra bands recorded for the Cu(II)-IIP and NIP polymers are similar indicating that the synthesis of Cu(II)-IIP was successful. The only difference was an additional IR band at 1550–1610 cm−1 present for Cu(II)-IIP, which originates from Cu–O bond and indicates formation of a salt of copper(II) and the itaconic acid. The precise structure of the salt is unknown and need to be determined by more sophisticated analytical methods (e.g., NMR).
To confirm that the copper ions were incorporated in the polymer network, the WD-XRF spectra of Cu(II)-IIP and NIP were recorded (Fig. 3).
It can be observed that an intense signal of copper appears only for Cu(II)-IIP.
To evaluate the concentration of Cu(II) in the synthesized polymers, a quantitative analysis was performed. The concentration of copper was found to be 21.5 mg g−1 and was below the detection limit in the Cu(II)-IIP and NIP, respectively.
Surface area measurement
The surface area of IIP and NIP was calculated using the Eq. 4 [49].
where A s is the imprinted polymer surface area in m2 g−1, G is the amount of methylene blue adsorbed (g), N AV is the Avogadro’s number (6.02 × 1023 mol−1), Ø is the methylene blue molecular cross section (197.2 Å2), M W is the molecular weight of methylene blue (373.9 g mol−1), M is the mass of adsorbent (g).
The calculated surface area of leached Cu(II)-IIP and NIP was 14.56 and 11.65 m2 g−1, respectively.
Effect of pH
One of the most critical parameters influencing adsorption of metal ions is pH of the solution. Thus, the effect of varying pH values on Cu(II) uptake was investigated using a batch procedure. The binding capacity of Cu(II)-IIP and NIP was evaluated in the pH range of 2.09–6.09. In consideration of hydrolysis, pH above 6.09 was not tested [31].
As can be observed in Fig. 4, the adsorption capacity of Cu(II) increases with the pH increasing, reaching the maximum at pH 6.0. Below pH 4.0, the adsorption capacity was low due to the protonation of the polymers. This occurrence was in a good agreement with the data reported in the literature [22, 30, 35]. The optimum pH exploited for further experiments was 6.0.
Adsorption properties
One of the most important properties characterizing the ion-imprinted polymers is the adsorption capacity (Q), defined as the total amount of ion adsorbed per gram of the sorbent particles [50].
Static adsorption capacity
The static adsorption capacity is the maximum concentration of ions that can be adsorbed by the polymer at equilibrium state [51]. The adsorption of copper (II) ions from the metal ion-containing aqueous solution was investigated. The static adsorption capacity for Cu(II)-IIP and NIP was 14.8 and 4.08 mg/g, respectively. The adsorption capacity of the polymer reported in the previous publications was in the following range: 0.3–28 mg/g [21–23, 25, 26, 35, 36, 38, 57]. Only one polymer characterized in the literature had a higher value of the Q parameter (Q = 76 mg/g) [37]. It should be pointed out that the majority of authors determined the adsorption capacity only for the copper ion-imprinted polymers. They have not compared this result with the adsorption capacity of the non-imprinted polymers. The question arises as to what we can actually discover from these data? Is the adsorption capacity of the imprinted polymer higher than that of the control polymer? Do the synthesized imprinted polymers have in their structure the specific cavities that are able do adsorb only the copper ion?
The adsorption capacity of our Cu(II)-IIP is 3.6 times higher than that of NIP which, according to the data available in the literature, is the highest value [21].
Adsorption kinetics
The adsorption properties of IIPs can be evaluated by the adsorption isotherms in the batch experiments. These isotherms provide a relationship between the concentration of the analyte metal ion in a solution and that adsorbed on the solid sorbent, when the two phases are in equilibrium. The Langmuir adsorption isotherm model was used to evaluate the adsorption properties of the particles of Cu(II)-IIP. This model is a theoretical equation that is applicable to homogeneous binding sites and assumes that the molecules are adsorbed at a fixed number of well-defined sites, each of which can only hold one molecule. These sites are also assumed to be energetically equivalent and distant to each other so that there are no interactions between molecules adsorbed on adjacent sites [52, 53].
The Langmuir adsorption isotherm is expressed by the Eq. 4 [24, 29, 54].
where C e is the equilibrium concentration of metal ions (mg/L), Q is the amount of metal ions adsorbed (mg/g), Q m is the maximum adsorption capacity of metal ions (mg/g), and b is the Langmuir adsorption equilibrium constant (L/mg) [55, 56].
Figure 5 shows the dependence of the equilibrium concentration on the adsorbed amount of Cu2+ onto the Cu(II)-IIP particles.
The equilibrium adsorption data of the Cu(II) ions were highly correlated according to the Langmuir adsorption model (R 2 = 0.9969), which indicates that the adsorption obeys the Langmuir model assumptions. The maximum adsorption capacity Q m was 16.12 mg/g.
Selectivity studies
To evaluate the selectivity of the imprinted polymer, the competitive sorption of Cu2+/Ni2+ and Cu2+/Zn2+ from their binary mixtures was investigated in batch experiments. It is worth mentioning that the interfering ions have the same charge and similar ion radii with copper (II) ion (Cu2+—71 pm, Ni2+—69 pm, Zn2+—74 pm) [38]. The results in Table 2 indicate that the relative selectivity coefficients of the Cu(II)-IIP increased with increasing ratio of Cu2+/M2+. Depending on the interfering ion and its concentration referring to Cu2+, the enrichment factors were 3.30–32.4 times greater than those of the non-imprinted polymers. It indicates that the synthesized copper(II) ion-imprinted polymer can selectively adsorb copper ions in the presence of interfering ions.
Table 3 shows a comparison of the values of the adsorption capacity and relative selectivity coefficients of the new Cu(II)-IIP and polymers reported in the literature.
Although the adsorption capacity of the polymer characterized in this work is lower than other copper(II) ion-imprinted polymers [31, 37, 57], the data presented in Table 3 indicate that it is showing the best selectivity towards Ni2+ ions and very high selectivity towards Zn2+ ions.
Conclusions
This work reports the preparation and characterization of a new copper(II) ion-imprinted polymer. Prior to the precipitation polymerization, the compound of copper(II) and itaconic acid was prepared in methanol. The cross-linking monomer and an initiator were ethylene glycol dimethacrylate and 2,2′-azobisisobutyronitrile, respectively.
It should be pointed out that in this work WD-XRF method was first applied do determine the copper (II) content directly in the ion-imprinted polymer matrix, which allows checking, e.g., the effectiveness of leaching of the Cu(II) ions from the synthesized polymer. Furthermore, it enables to determine of the copper ions concentration in the polymer after synthesis.
The new Cu(II)-imprinted polymer exhibits good characteristics for adsorption of Cu2+ and shows very good selectivity towards Zn2+ and Ni2+ ions. It is worth mentioning that among other copper ion-imprinted polymers, the new Cu(II)-IIP exhibits the best selectivity towards the nickel ion. Therefore, it can be successfully applied for the enrichment and selective adsorption of copper (II) ion from the samples of a complex matrix (e.g., biological fluids, environmental samples) prior to their analysis by the spectroscopic methods.
References
World Health Organization (2011) Guidelines for drinking-water quality, 4th edn. p 224
Uauy R, Olivares M, Gonzalez M (1998) Essentiality of copper in humans. Am J Clin Nutr 67:952–959
Turnlund JR (2006) In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ (eds) Modern nutrition in health and disease, 10th edn. Lippincott Williams & Wilkins, Philadelphia, p 286
Johnson MA, Fischer JG, Kays SE (1992) Is copper an antioxidant nutrient? Crit Rev Food Sci Nutr 32(1):1–31
Angelova M, Asenova S, Nedkova V, Koleva-Kolarova R (2011) Copper in the Human Organism. TJS 9(1):88–98
Desai V, Kaler SG (2008) Role of copper in human neurological disorders. Am J Clin Nutr 88:855S–858S
Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Jalbani N, Kandhro GA (2008) Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res 122(1):1–18
Akman S, Welz B, Tokman N (2005) Investigation of interference mechanisms of nickel chloride on copper determination and of colloidal palladium as modifier in electrothermal atomic absorption spectrometry using a dual cavity platform. Spectrochim Acta Part B 60:1349–1356
Shrivas K (2010) Monitoring of copper level in water and soil samples by using liquid–liquid extraction. Environ Monit Assess 168:315–319
Farajzadeh MA, Bahram M, Zorita S, Mehr BG (2009) Optimization and application of homogeneous liquid-liquid extraction in preconcentration of copper (II) in a ternary solvent system. J Hazard Mater 161(2–3):1535–1543
Citak D, Tuzen M, Soylak M (2009) Simultaneous coprecipitation of lead, cobalt, copper, cadmium, iron and nickel in food samples with zirconium (IV) hydroxide prior to their flame atomic absorption spectrometric determination. Food Chem Toxicol 47:2302–2307
Escudero LA, Cerutti S, Olsina RA, Salonia JA, Gasquez JA (2010) Factorial design optimization of experimental variables in the on-line separation/preconcentration of copper in water samples using solid phase extraction and ICP-OES determination. J Hazard Mater 183:218–223
Dindar MH, Fathi SAM, Yaftian MR, Noushiranzadeh N (2010) Solid phase extraction of copper(II) ions using C18-silica disks modified by oxime ligands. J Hazard Mater 179:289–298
Yamini Y, Faraji M, Shariati S, Hassani R, Ghambarian M (2008) On-line metals preconcentration and simultaneous determination using cloud point extraction and inductively coupled plasma optical emission spectrometry in water samples. Anal Chim Acta 612:144–151
Ghaedi M, Shokrollahi A, Ahmadi F, Rajabi HM, Soylak M (2008) Cloud point extraction for the determination of copper, nickel and cobalt ions in environmental samples by flame atomic absorption spectrometry. J Hazard Mater 150:533–540
Liska I (1993) On-line versus off-line solid-phase extraction in the determination of organic contaminants in water. J Chromatogr A 655:163–176
Rao TP, Daniel S, Gladis JM (2004) Tailored materials for preconcentration or separation of metals by ion imprinted polymers for solid-phase extraction (IIP-SPE). Trends Anal Chem 23(1):28–35
Nishide H, Deguchi J, Tsuchida E (1976) Selective adsorption of metal ions on crosslinked poly(vinylpyridine) resin prepared with a metal ions as a template. Chem Lett 5:169–176
Nishide H, Deguchi J, Tsuchida E (1977) Adsorption of metal ions on crosslinked poly(4-vinylpyridine) resins prepared with a metal ion as template. J Polym Sci Polym Chem 15:3023–3029
Branger C, Meouche W, Margaillan A (2013) Recent advances on ion-imprinted polymers. React Funct Polym 73:859–875
Singh DK, Mishra S (2009) Synthesis of a new Cu (II)-ion imprinted polymer for solid phase extraction and preconcentration of Cu (II). Chromatographia 70:1539–1544
Shamsipur M, Fasihi J, Khanchi A, Hassani R (2007) A stoichiometric imprinted chelating resin for selective recognition of copper (II) ions in aqueous media. Anal Chim Acta 599:294–301
Dakova I, Karadjova I, Ivanov I, Georgieva V (2007) Solid phase selective separation and preconcentration of Cu(II) by Cu(II)-imprinted polymethacrylic microbeads. Anal Chim Acta 584:196–203
Hoai NT, Yoo DK, Kim D (2010) Batch and column separation characteristics of copper-imprinted porous polymer micro-beads synthesized by a direct imprinting method. J Hazard Mater 173:462–467
Walas S, Tobiasz A, Gawin M, Trzewik B (2008) Application of a metal ion-imprinted polymer based on salen–Cu complex to flow injection preconcentration and FAAS determination of copper. Talanta 76:96–101
Tobiasz A, Walas S, Trzewik B, Grzybek P (2009) Cu(II)-imprinted styrene-divinylbenzene beads as a new sorbent for flow injection-flame atomic absorption determination of copper. Microchem J 93:87–92
Bhunia A, Roesky PW, Lan Y, Kostakis GE (2009) Salen-based infinite coordination polymers of nickel and copper. Inorg Chem 48:10483–10485
Bhunia A, Gotthardt MA, Yadav M, Gamer MT (2013) Salen-based coordination polymers of manganese and the rare-earth elements: synthesis and catalytic aerobic epoxidation of olefins. Chem Eur J 19:1986–1995
Hoai NT, Kim D (2009) Synthesis, structure, and selective separation behavior of copper-imprinted microporous polymethacrylate beads. AIChE 55:3248–3254
Kanazawa R, Mori K, Tokuyama H, Sakohara S (2004) Preparation of thermosensitive microgel adsorbent for quick adsorption of heavy metal ions by a temperature change. J Chem Eng Jpn 37:804–807
Shamsipur M, Besharati-Seidani A, Fasihi J, Sharghi H (2010) Synthesis and characterization of novel ion-imprinted polymeric nanoparticles for very fast and highly selective recognition of copper (II) ions. Talanta 83:674–681
Ebrahimzadeh H, Behbahani M, Yamini Y, Adlnasab L (2013) Optimization of Cu(II)-ion imprinted nanoparticles for trace monitoring of copper in water and fish samples using a Box-Behnken design. React Funct Polym 73:23–29
Baghel A, Boopathi M, Singh B, Pandey P, Mahato TH, Gutch PK, Sekhar K (2007) Synthesis and characterization of metal ion imprinted nano-porous polymer for the selective recognition of copper. Biosens Bioelectron 22:3326–3334
Ng SM, Narayanaswamy R (2010) Demonstration of a simple, economical and practical technique utilising an imprinted polymer for metal ion sensing. Microchim Acta 169:303–311
Zhai Y, Yang D, Chang X, Liu Y (2008) Selective enrichment of trace copper(II) from biological and natural water samples by SPE using ion-imprinted polymer. J Sep Sci 31:1195–1200
Zhai Y, Liu Y, Chang X, Chen S (2007) Selective solid-phase extraction of trace cadmium(II) with an ionic imprinted polymer prepared from a dual-ligand monomer. Anal Chim Acta 593:123–128
Wang S, Zhang R (2006) Selective solid-phase extraction of trace copper ions in aqueous solution with a Cu(II)-imprinted interpenetrating polymer network gel prepared by ionic imprinted polymer (IIP) technique. Microchim Acta 154:73–80
Dam AH, Kim D (2008) Synthesis, structure, and selective separation behavior of copper-imprinted microporous polymethacrylate beads. J Appl Polym Sci 108:14–24
Say R, Birlik E, Ersöz A, Yılmaz F, Gedikbey T, Denizli A (2003) Preconcentration of copper on ion- selective imprinted polymer microbeads. Anal Chim Acta 480:251–258
Shamsipur M, Besharati-Seidani A (2011) Synthesis of a novel nanostructured ion-imprinted polymer for very fast and highly selective recognition of copper(II) ions in aqueous media. React Funct Polym 71:131–139
Lopes Pinheiro SC, Descalzo AB, Raimundo IM Jr, Orellana G, Moreno-Bondi MC (2012) Fluorescent ion-imprinted polymers for selective Cu(II) optosensing. Anal Bioanal Chem 402:3253–3260
Cui Ch, He M, Chen B, Hu B (2013) Restricted accessed material-copper(II) ion imprinted polymer solid phase extraction combined with inductively coupled plasma-optical emission spectrometry for the determination of free Cu(II) in urine and serum samples. Talanta 116:1040–1046
Compton TR (2007) Determination of metals in: determination of additives in polymers and rubbers. iSmithers Rapra Publishing
Bichinho KM, Pozzobon Pires G, Stedile FC, dos Santos JHZ, Wolf CR (2005) Determination of catalyst metal residues in polymers by X-ray fluorescence. Spectrochimica Acta Part B 60:599–604
Bianchini D, Bichinho KM, dos Santos JHZ (2002) Polyethylenes produced with zirconocene immobilized on MAO-modified silicas. Polymer 43:2937–2943
Cook WS, Jones CO, Altenau AG (1968) X-ray fluorescence analysis of additive elements in polymers. Can Spectr 13:64–68
Meites L (1963) Handbook of analytical chemistry. McGraw-Hill Book Company, New York
Nacano LR, Segatelli MG, Tarley CRT (2010) Selective sorbent enrichment of nickel ions from aqueous solutions using a hierarchically hybrid organic-inorganic polymer based on double imprinting concept. J Braz Chem Soc 21:419–430
Kaewprasit C, Hequet E, Abidi N, Gourlot JP (1998) Application of methylene blue adsorption to cotton fiber specific surface area measurement: part I. Methodology. J Cotton Sci 2:164–173
Shakerian F, Dadfarnia S, Haji Shabani AM (2012) Synthesis and application of nano-pore size ion imprinted polymer for solid phase extraction and determination of zinc in different matrices. Food Chem 134:488–493
Tsoi YK, Ho YM, Sze-Yin Leung K (2012) Selective recognition of arsenic by tailoring ion-imprinted polymer for ICP-MS quantification. Talanta 89:162–168
Hasan S, Krishnaiah A, Ghosh TK, Viswanath DS, Boddu VM, Smith ED (2003) Adsorption of chromium(VI) on chitosan-coated perlite. Sep Sci Technol 38:3775–3793
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Chen AH, Yang CY, Chen CY, Chen CY, Chen CW (2009) The chemically crosslinked metal-complexed chitosans for comparative adsorptions of Cu(II), Zn(II), Ni(II) and Pb(II) ions in aqueous medium. J Hazard Mat 163:1068–1075
Niu C, Wu W, Wang Z, Li S, Wang J (2007) Adsorption of heavy metal ions from aqueous solution by crosslinked carboxymethyl konjac glucomannan. J Hazard Mater 141:209–214
Ng JCY, Cheung WH, McKay G (2003) Equilibrium studies for the sorption of lead from effluents using chitosan. Chemosphere 52:1021–1030
Jiang Y, Kim D (2011) Effect of solvent/monomer feed ratio on the structure and adsorption properties of Cu2+-imprinted microporous polymer particles. Chem Eng J 166:435–444
Acknowledgments
The authors wish to thank Dr. Piotr Goś for his valuable advices concerning the polymers preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kuras, M.J., Więckowska, E. Synthesis and characterization of a new copper(II) ion-imprinted polymer. Polym. Bull. 72, 3227–3240 (2015). https://doi.org/10.1007/s00289-015-1463-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1463-8