Abstract
A novel initiator, naturally occurring in soy, has been developed for a direct synthesis of aliphatic star-shaped homo- and copolyesters. The synthesis is based on one of several known isoflavones, genistein, which has been employed as an initiator of homopolymerization of poly(l-Lactide) or co-initiator of copolymerization of ε-caprolactone and l-Lactide with Sn(Oct)2 as a catalyst. The non-toxic chemicals used allow for an inexpensive and safe for human body facile synthesis of biocompatible functionalized polymers suitable for medical and pharmaceutical applications. The obtained polymers were characterized by 1H, 13C NMR, FT-IR, SEC–MALLS and MALDI-TOF MS analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considerable interest exists in the synthesis of aliphatic homo- and copolyesters for their potential applications as biomedical materials, such as controlled drug delivery systems, surgical sutures and internal bone fixation [1–4]. Poly(l-Lactide) (PLA) and poly(ε-caprolactone) (PCL) as well as their copolymers are the most extensively studied, characterized and used as biomedical materials due to their biodegradability, biocompatibility and permeability [5, 6]. Polyesters with well-defined architectures such as star-shaped, comb-like and dendritic polymers with controlled chain numbers and lengths are attracting interest because they can modify structures and physical properties of PLA and PCL (e.g., their high degree of crystallinity) [7, 8]. These homo- and copolymers are usually synthesized by the ring-opening polymerization (ROP) and copolymerization of l-Lactide (LLA) or ε-caprolactone (CL) in the presence of multifunctional initiators containing hydroxyl or amine groups.
In recent years, many approaches have been made to optimize the properties of the obtained polymers, such as their bioactivity and biocompatibility, to satisfy the requirements of specific biomedical applications. A promising example is incorporation of bioactive or biocompatible hormones, peptides, amino acids, lipids and other natural compounds into the polymer chain [9–12]. Recently, Dong and coworkers investigated the synthesis, crystallization and morphology of six-arm star-shaped PCL obtained by the ROP of CL with dipentaerythritol as the initiator in bulk [13]. In their laboratory, polypseudorotaxanes composed of star-shaped porphyrin-cored PCL and α-cyclodextrin as well as a new class of linear-dendron-like PCL-b-poly(ethylene oxide) copolymers have also been successfully synthesized and characterized in detail [14, 15]. Furthermore, Cho et al. [16] studied the details of the synthesis of star-shaped, amphiphilic block copolymers composed of fully degradable PCL by initiation with pentaerythritol, while Cui et al. [17] reported the synthesis and characterization of copolymers of CL and LLA using cyclotriphosphazene core. As an extension, star-shaped PCLs with PAMAM dendrimer core have been successfully synthesized and characterized in our laboratory [18].
Genistein (Gns) is one of the isoflavones that can be found in soy [19]. Recently, it has been reported that this compound has weak estrogenic and antiestrogenic properties, is an antioxidant, inhibits topoisomerase II and angiogenesis and induces cell differentiation [19]. Furthermore, it has been found that Gns promotes cell differentiation resulting in a less active epidermal growth factor signaling pathway in adulthood that, in turn, suppresses the development of mammary cancer [20].
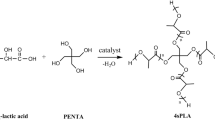
As a matter of fact, it can also be a suitable material for the synthesis of star-shaped polymers due to the presence of three hydroxyl groups in the molecule, enabling the initiation of ROP of LLA or CL. Therefore, it is reasonable to prepare homo- and copolymers with the desired biomedical properties inherited from the Gns molecule. In the ongoing study, biodegradable and biocompatible PLA homopolymers and random copolymers of LLA and CL were synthesized and characterized using for the first time naturally occurring Gns as a core. The influence of the Gns molecule on the thermal properties and hydrolysis stability of the obtained polyesters, as well as the release profile of the covalently conjugated drug to the resulting polymers, will be discussed in the next paper.
Experimental
Materials
ε-Caprolactone (2-oxepanone, ≥99.0 %, Aldrich Co. Poland) was dried and distilled before use over CaH2 at reduced pressure. l-Lactide ((3S)-cis-3,6-dimethyl-1,4-dioxane-2,5-dione, 98.0 %, Aldrich Co. Poland) was recrystallized from dried ethyl acetate in a dry nitrogen atmosphere and then thoroughly dried in vacuum before use. Genistein (Gns, 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, ≥98 %, Sigma Co. Poland) was dried before use under vacuum at 40 °C for 4 h. Stannous octoate (Sn(Oct)2, tin(II) 2-ethylhexanoate, 2-ethylhexanoic acid tin(II) salt, ~95 %, Aldrich Co. Poland) was used as received. Dichloromethane (anhydrous, ≥99.8 %, POCh, Gliwice, Poland), methanol (anhydrous, 99.8 %, POCh, Gliwice, Poland) and diethyl ether (anhydrous, 99.8 %, POCh, Gliwice, Poland) were used as received.
Measurements
The polymerization products were characterized in the DMSO–d6 solution by means of 1H and 13C NMR techniques (Varian 300 MHz). The FT-IR spectra in the 400–4,000 cm−1 range were measured from KBr pellets using Spectrum 1,000 spectrometer (Perkin–Elmer).
Number-average molecular weights (M n ) and polydispersity indexes (M w /M n ) were measured using SEC–MALLS instrument composed of an 1,100 Agilent isocratic pump, autosampler, degasser, thermostatic box for columns, a photometer MALLS DAWN EOS (Wyatt Technology Corporation, Santa Barbara, CA) and differential refractometer Optilab Rex. ASTRA 4.90.07 software (Wyatt Technology Corporation) was used for data collecting and processing. Two 2 X TSKgel MultiporeHXLcolumns were used for separation. The samples were injected as a solution in methylene chloride. The volume of the injection loop was 100 μL. Methylene chloride was used as a mobile phase at a flow rate of 0.8 mL min−1.
Mass spectrometric measurements were performed using the ultrafleXtreme™ MALDI-TOF MS spectrometer (Bruker Daltonics, Fremont, CA) equipped with a pulsed N2 laser and a time-delayed extraction ion source. An acceleration voltage of 20 kV was used. Mass spectra were obtained in the linear mode. The matrix 2[(4-hydroxyphenyl)diazenyl] benzoic acid (HABA) was dissolved in THF or methylene chloride at a concentration equal to 10 mg mL−1 and mixed with a polymer sample in a 25:1 v/v ratio. NaI was added as a cationizing agent. The mixture was dried on stainless steel plate covered by the gold metal target.
Polymerization procedure
The polymeric materials were prepared using different molar ratios of initiator (genistein (Gns) to monomers (ε-caprolactone (CL) or l-Lactide (LLA). The initiator/monomer feed ratios for the received polymers were: 1/25; 1/50; 1/75 (mol/mol) for homopolymers of PLA and 1/25/25; 1/10/40; 1/40/10 for copolymers of PLA/PCL denoted as Gns/PLA 25; Gns/PLA 50; Gns/PLA 75; Gns/PLA25/PCL25; Gns/PLA10/PCL40; Gns/PLA40/PCL10; respectively, where Gns = genistein; PLA = poly(l-Lactide) and PCL = poly(ε-caprolactone). For the homopolymerization, dry Gns and LLA were accurately weighed and introduced into a 50 mL polymerization tube. The tube was then connected to a Schlenk line, where exhausting–refilling processes were repeated three times. The tube was immersed into an oil bath at 140 °C under argon atmosphere for 48 h. After an appropriate time, the reaction products were cooled down, dissolved in dry CH2Cl2, precipitated twice from a cold diethyl ether and dried under vacuum for 72 h.
NMR data for Gns/PLA 25
1H NMR (DMSO-d6, 300 MHz, δ H, ppm); 8.31 (s, H-1), 7.38–7.35 (dd, H-1′, 4′, J 1′, 2′ = 8.5, J 1′, 4′ = 2.0,), 6.82–6.80 (dd, H-2′, 3′, J 1′, 2′ = 8.5, J 1′, 3′ = 2.0), 6.38 (d, H-6, J 6, 5 = 2.0), 6.24 (d, H-5, J 6, 5 = 2.0), 5.18 (q, –CH(CH3)– of PLA (a)), 4.97 (q, –CH(CH3)– of PLA (a ′′)), 4.18 (q, –CH(CH3), end group of PLA (a′)), 1.45 (d, –CH 3 of PLA (b + b′′)), 1.27 (d, –CH 3 , end group of PLLA (b′) (Fig. 1a and Fig. S-1, electronic supplementary material).
13C-NMR (DMSO-d6, 300 MHz, δ, ppm); 13C-NMR (DMSO-d6, 300 MHz, δ, ppm); 181.12 (4), 173.98 (c′), 171.14 (c′′), 169.16 (c), 168.11 (4′ + 5 + 7), 158.51 (9), 153.90 (2), 131.15 (2′ + 6′), 124.50 (3 + 1′), 115.02 (3′ + 5′), 106.00 (10), 98.92 (6), 93.36 (8), 68.64 (a + a′′), 65.56 (a′), 16.43 (b + b′ + b′′) (Figs. S-2 and S-3, electronic supplementary material).
FT-IR data for Gns/PLA 25
FT-IR (KBr, cm−1): 2,995 (υ asCH3), 2,947 (υ sCH3), 2,884 (υCH), 1,747 (υC = O), 1,451 (δ asCH3), 1,378 (δ sCH3), 1,368–1,360 (δ 1CH + δ sCH3), 1,264 (δCH + υCOC), 1,197 (υ asCOC + rasCH3), 1,131 (rasCH3), 1,091 (υ sCOC), 1,045 (υC-CH3), 952 (rCH3 + υCC), 863 (υC-COO), 754 (δC = 0), 670 (γC = O) (Fig. S-5, electronic supplementary material).
For the copolymerization, dry Gns and LLA were accurately weighed and introduced into 50 mL polymerization tube. The tube was then connected to a Schlenk line, where exhausting–refilling processes were repeated three times. The tube was immersed into an oil bath at 140 °C under an argon atmosphere for 48 h. After an appropriate time, the precise weight of CL and a catalytic amount of Sn(Oct)2 were added to the melted mixture and the exhausting–refilling process was carried out again. The polymerization tube was put into an oil bath at 110 °C under nitrogen atmosphere and cooled to room temperature after polymerization for 24 h. The resulting products were dissolved in dry CH2Cl2, precipitated twice from a cold diethyl ether and dried under vacuum for 72 h.
NMR data for Gns/PLA25/PCL25
1H NMR (DMSO-d6, 300 MHz, δ H, ppm); 8.32 (s, H-1), 7.38–7.35 (dd, H-1′, 4′, J 1′, 2′ = 8.5, J 1′, 4′ = 2.0,), 6.82–6.79 (dd, H-2′, 3′, J 1′, 2′ = 8.5, J 1′, 3′ = 2.0), 6.38 (d, H-6, J 6, 5 = 2.0), 6.21 (d, H-5, J 6, 5 = 2.0), 5.19 (q, –CH(CH3)– of PLA (a)), 4.94 (q, –CH(CH3)– of PLA (a ′′)), 4.32 (q, –CH(CH3) of PLA (a′)), 4.06 (t, -CH 2 O- of PCL (b′)), 3.97 (t, -CH 2 O- of PCL (b)), 3.31 (t, –CH 2 OH, end group of PCL (b′′)), 2.32 (t, –CH2CH 2 C(O)– of PCL (c′)), 2.26 (t, –CH2CH 2 C(O)– of PCL (c)), 1.57–1.40 (m, –CH 2 CH2C(O)– of PCL (d) and –CH 3 of PLA (f + f′ + f′′)), 1.29 (t, –CH2CH 2 CH2– of PCL (e) (Fig. 1b, Fig. S-1, electronic supplementary material).
13C-NMR (DMSO-d6, 300 MHz, δ, ppm); 181.14 (4), 173.91 (a′), 173.37 (h), 172.04 (h′), 169.65 (a), 168.37 (4′ + 5 + 7), 158.61 (9), 153.94 (2), 130.09 (2′ + 6′), 124.77 (3,1′), 115.01 (3′ + 5′), 106.09 (10), 98.91 (6), 93.61 (8), 68.14 (b + b′′), 67.70 (b′), 64.42 (e′), 63.43 (e), 61.08 (e′′), 33.31 (d), 61.08 (d′), 27.72 (f), 24.69 (f), 24.04 (g), 16.46 (c + c′ + c′′) (Figs. S-2 and S-4, electronic supplementary material).
FT-IR data for Gns/PLA25/PCL25
FT-IR (KBr, cm−1): 2,997 (υ asCH3), 2,949–2,945 (υ asCH2) (υ sCH3), 2,865 (υ sCH2), 1,764 (υC = O), 1,457 (δasCH3), 1,348–1,387 (δsCH3), 1,368–1,360 (δ1CH + δsCH3), 1,315–1,300 (δ2CH),1,275 (υasCOC), 1,161 (υsCOC) (Fig. S-6, electronic supplementary material).
Results and discussion
The ROP homopolymerization of LLA was carried out in bulk in the presence of natural isoflavonoid Gns, identified as an angiogenesis inhibitor [19]. To obtain LLA prepolymers with different molecular weights, the molar ratio of LLA and the initiator Gns was varied ([LLA]/[I] = 25, 50, 75). The 1H NMR spectrum of Gns/PLA 25 is shown in Fig. 1a.
The major resonance signals a and b + b′ were attributed to the PLA chain, whereas signal a′ to the methine proton signal terminated by the hydroxyl end group. Furthermore, signal a′′ was assigned to the methine proton signal of PLA unit directly connected to the initiator molecule. The most important, the proton signals of phenyl and benzopyran-4-one rings of Gns (Fig. S-1, electronic supplementary material) were also detected with high resolution, indicating incorporation of Gns into the macromolecule (Fig. 1a) [21]. 1H NMR analysis demonstrated that naturally occurring Gns successfully initiated ROP of LLA. An additional confirmation of the presence of Gns in the macromolecule is given by comparison of the 13C NMR spectra of the resulting homopolymer and the pure initiator (Figs. S-2 and S-3, electronic supplementary material).
Based on the generally accepted conclusion, the number-average molecular weights determined by 1H NMR spectroscopy (M n(NMR)) were calculated from the average chain length (DP) and average degree of substitution (DS) of the resulting homopolymers [22]. The calculation results as well as SEC–MALLS data are shown in Table 1.
The results revealed that the three PLA arms can be attached to the Gns initiator molecule, demonstrating that the three hydroxyl groups of Gns were effective initiation centers of ROP of LLA. Furthermore, it was noted that M n obtained by SEC–MALLS were similar to those calculated from 1H NMR analysis and that polymer materials with high yield and moderate molecular weight distribution were prepared using the natural initiator.
The hydroxyl end-capped PLAs with Gns core were then applied as macroinitiators of ROP of CL (Scheme 1). The copolymerization was carried out in bulk at moderate temperature around 110 °C to avoid transesterification [23, 24]. SEC–MALLS analysis was carried out for PLA macroinitiator and then for product copolymerization. The typical SEC–MALLS curves of the resulting products revealed a symmetrical elution peak with narrow polydispersity (M w /M n , Table 1). Furthermore, an expected shift to higher molar masses was observed for the PLA-r-PCL copolymer when compared with PLA macroinitiator (Fig. 2; Table 1).
The 1H NMR spectrum of Gns/PLA25/PCL25 is shown in Fig. 1b. The signals assigned to the phenyl and benzopyran-4-one protons rings of Gns may be clearly observed [as comparison see the 1H NMR spectrum of the pure initiator (Fig. S-1, electronic supplementary material)]. Moreover, the presence of a new triplet signal assigned to methylene protons (b′′) indicated that the resulting copolymers were produced with PCL blocks as end groups and that the terminal hydroxyl groups of the macroinitiators effectively initiated the polymerization of CL. This occurrence is in accordance with our previous study [25]. Figure S-4 (electronic supplementary material) shows the 13C NMR spectra of Gns/PLA25/PCL25 that additionally confirm the structure of the resulting copolymers. The number-average molecular weights, the average chain length as well as degree of substitution were also determined by 1H NMR spectroscopy. The calculation results suggested a similar trend to that observed in the PLA system. The molecular weight distribution was also moderate and the number-average molecular weights obtained by SEC–MALLS analysis were similar to 1H NMR calculations (Table 1).
Figure 3 shows the MALDI-TOF mass spectrum of PLA obtained from the homopolymerization of LLA initiated by Gns (Gns/PLA 25).
The spectrum shows four main series of peaks. The first series corresponds to a PLA molecule terminating with one hydroxyl and one hydrogen end group (residual mass 41, Na+ adduct, A). The second series of peaks can be assigned to PLA terminating with Gns and hydrogen end groups (residual mass 271, H+ adduct, B). The third series, with smaller intensity, also corresponds to PLA terminating with Gns and hydrogen end groups, but with Na+ adduct (residual mass 274, C), while the fourth weak series of peaks is consistent with macrocyclic polymers (residual mass 3, Na+ adduct, D).
In the mass spectrum in Fig. 3, two populations of chains were detected that is with even and odd number of LA repeating units. They were separated by 72 amu. The two populations can be explained as typical of LLA polymerization and intra- and intermolecular transesterification reaction [25].
The MALDI-TOF mass spectrum result clearly demonstrates the presence of the Gns molecule in the macromolecule that indicates quite effective initiation properties of Gns in the ROP of LLA. The formation of the polymer chains terminated with hydroxyl and hydrogen end groups (series A, Fig. 3) might be explained by hydrolysis process that can be usually occurred during precipitation of the polymer (in this study methylene chloride and diethyl ether/water environment) or concerning MALDI-TOF MS measurements as a result of mixing of the polymer sample with the matrix materials [26, 27], whereas formation of macrocyclic subunits by an intramolecular transesterification that favorable appeared under polymerization conditions [23].
Conclusions
In summary, to the best of my knowledge, this is the first report that describes the homo- and copolymerization of cyclic esters initiated by the natural isoflavonoid-genistein. Polymerization in bulk produced polymeric materials with a moderate molecular weight up to 12,100 [g/mol] and yield as high as 98 %. Spectroscopic data have clearly indicated the incorporation of genistein into the macromolecule. Due to the use of non-toxic chemicals, the obtained results are promising for the synthesis of the star-shaped macromolecules and their application as drug carriers in pharmacy.
References
Albertsson AC, Varma IK (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4(6):1466–1486
Hu Z, Fan X, Wang H, Wang J (2009) Synthesis and characterization of biodegradable and biocompatible amphiphilic block copolymers bearing pendant amino acid residues. Polymer 50(17):4175–4181
Oh JK (2011) Polylactide (PLA)-based amphiphilic block copolymers: synthesis, self-assembly, and biomedical applications. Soft Matter 7(11):5096–5108
He Ch, Kim SW, Lee DS (2007) In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release 127(3):189–207
Broström J, Boss A, Chronakis IS (2004) Biodegradable films of partly branched poly(l-lactide)-co-poly(ε-caprolactone) copolymer: modulation of phase morphology, plasticization properties and thermal depolymerization. Biomacromolecules 5(3):1124–1134
Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J (2005) Synthesis and evaluation of a star amphiphilic block copolymer from poly(ε-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconjugate Chem 16(2):397–405
Kikkawa Y, Abe H, Iwata T, Inoue Y, Doi Y (2002) Crystallization, stability, and enzymatic degradation of poly(l-lactide) thin film. Biomacromolecules 3(2):350–356
Persenaire O, Alexandre M, Degée P, Dubois P (2001) Mechanisms and kinetics of thermal degradation of poly(ε-caprolactone). Biomacromolecules 2(1):288–294
Meneghetti SP, Lutz PJ, Rein D (1999) Star-shaped polymers via anionic polymerization methods. In: Mishra MK, Kobayashi S (eds) Star and hyperbranched polymers, Marcel Dekker, New York, pp. 27–57
Cameron DJA, Shaver MP (2011) Aliphatic polyester polymer stars: synthesis, properties and applications in biomedicine and nanotechnology. Chem Soc Rev 40:1761–1776
Oledzka E, Sobczak M (2012) Polymers in the pharmaceutical applications—natural and bioactive initiators and catalysts in the synthesis of biodegradable and bioresorbable polyesters and polycarbonates. In: Agbo EC (ed) Innovations in biotechnology, InTech, Croatia, pp. 139–160
Oledzka E, Sokolowski K, Sobczak M, Kołodziejski WL (2011) α-Amino acids as initiators of ε-caprolactone and L, l-lactide polymerization. Polym Int 60(5):787–793
Wang J-L, Wang L, Dong Ch-M (2005) Synthesis, crystallization and morphology of star-shaped poly(ε-caprolactone). J Polym Sci A Polym Chem 43:5449–5457
Dai X-H, Dong Ch-M, Fa H-B, Yan D, Wei Y (2006) Supramolecular polypseudorotaxanes composed of star-shaped porphyrin-cored poly(ε-caprolactone) and α-cyclodextrin. Biomacromolecules 7:3527–3533
Hua Ch, Peng S-M, Dong Ch-M (2008) Synthesis and characterization of linear-dendron-like poly(ε-caprolactone)-b-poly(ethylene oxide) copolymers via the combination of ring-opening polymerization and click chemistry. Macromolecules 41:6686–6695
An SG, Cho GCh (2004) Synthesis and characterization of amphiphilic poly(ε-caprolactone) star block copolymers. Macromol Rapid Comm 25(5):618–622
Cui Y, Tang X, Huang X, Chen Y (2003) Synthesis of the star-shaped copolymer of ε-caprolactone and l-lactide from a cyclotriphosphazene core. Biomacromolecules 4(6):1491–1494
Oledzka E, Kaliszewska D, Sobczak M, Raczak A, Nikel P, Kołodziejski W (2012) Synthesis and properties of a star-shaped poly(ε-caprolactone)–ibuprofen conjugate. J Biomat Sci Polym E 23(16):2039–2054
Doerge DR, Chang HC, Churchwell MI, Holder CL (2000) Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos 28:298–307
Lamartiniere CA (2000) Protection against breast cancer with genistein: a compound of soy. Am J Clin Nutr 71(6):1705–1707
Kolodziejski W, Mazurek AP, Kasprzycka-Guttman T (2000) 13C CP/MAS NMR study of a genistein/piperazine complex. Chem Phys Lett 328:263–269
Chang KY, Lee YD (2009) Ring-opening polymerization of ε-caprolactone initiated by the antitumor agent doxifluridine. Acta Biomater 5:1075–1081
Kricheldorf HR, Boettcher C, Tönnes KU (1992) Polylactones: 23. Polymerization of racemic and meso-d, Llactide with various organotin catalysts: stereochemical aspects. Polymer 33(13):2817–2824
Spassky N, Simic V, Montaudo MS, Hubert-Pfalzgraf LG (2000) Inter- and intramolecular ester exchange reactions in the ring-opening polymerization of (d, l-lactide using lanthanide alkoxide initiators. Macromol Chem Phys 201:2432–2440
Sobczak M, Kolodziejski W (2009) Polymerization of cyclic esters initiated by carnitine and tin (II) octoate. Molecules 14:621–632
Morrison RT, Boyd RK (1976)Organic Chemistry. In: Baes CF, Mesmer RE (eds) The hydrolysis of cations. 6th edns. Wiley, New York, pp 112–123
Kricheldorf HR, Eggerstedt S (1999) Macrocycles, 6. MALDI-TOF mass spectrometry of tin-initiated macrocyclic polylactones in comparison to classical mass-spectroscopic methods. Macromol. Chem Phys 200(6):1284–1291
Acknowledgments
The author is grateful for the financial support received from the Medical University of Warsaw.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
289_2013_973_MOESM1_ESM.doc
The online version of the article contains supplementary material, which is available to authorized users. Supplementary material 1 (DOC 1389 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Oledzka, E. Synthesis of genistein-containing star-shaped homo- and copolyesters by the ring-opening polymerization. Polym. Bull. 70, 2587–2597 (2013). https://doi.org/10.1007/s00289-013-0973-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-013-0973-5