Abstract
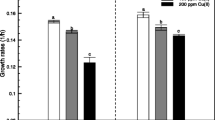
Intensifying sodic land characterized by high alkaline pH is an incipient environmental hazard-limiting agricultural potential. In this study, we investigated the effects of plant growth-promoting bacteria Ochrobactrum sp. strain NBRISH6 on the growth and physiology of maize (Zea mays L.) grown under alkaline stress at two soil pH levels. Additionally, we also studied the effects of NBRISH6 on soil fertility parameters. A greenhouse experiment was designed using two live soils (pH 8.2 and 10.2) in earthen pots using maize as a host. Results revealed a significant increase in plant growth and a decrease in defense enzymes in both soil types due to NBRISH6 inoculation as compared to non-treated control. Furthermore, activities of all soil enzymes along with bacterial diversity increased in NBRISH6 treatment under normal as well as stressed conditions. In addition, field evaluation of NBRISH6 inoculation using maize was carried out under normal and alkaline conditions, which resulted in significant enhancement of all vegetative parameters as compared to respective controls. Therefore, the study suggested that Ochrobactrum sp. NBRISH6 can be used to develop a bioinoculant formulation to ameliorate abiotic stresses and enhanced crop productivity.




Similar content being viewed by others
Code Availability
Not applicable.
References
Rengasamy P (2010) Soil processes affecting crop production in salt-affected soils. Fun Plant Biol 37:13–620
Liu J, Tang L, Gao H et al (2019) Enhancement of alfalfa yield and quality by plant growth promoting rhizobacteria under saline-alkali conditions. J Sci Food Agric 99:281–289
Damodaran T, Rai RB, Jha SK et al (2014) Rhizosphere and endophytic bacteria for induction of salt tolerance in gladiolus grown in sodic soils. J Plant Interact 9:577–584
Sapre S, Gontia-Mishra I, Tiwari S (2018) Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol Res 206:25–32
Khoshoo TN (1987) Ecodevelopment of alkaline land Banthara—a case study. NBRI, CSIR PID, New Delhi
Garg VK (2000) Bio-reclamation of sodic wasteland—a case study. Land Degrad Dev 11:163–172
Garg VK (2002) Sustainable rehabilitation of sodic soils through biological means—a case study. In: 12th ISCO Conference, Beijing, pp 149–155. http://www.tucson.ars.ag.gov/isco/isco12/VolumeIII/Sustainable.Rehabilitation.pdf
Singh PK, Kumar P, Tandon PK (2014) Soil sodicity alters antioxidative enzymes, photosynthestic pigments water content and essential oil quality of fennel (Foeniculum vulgare Mill). Res J Soil Biol 6:1–16
Fa-Hu LI, Keren R (2009) Calcareous sodic soil reclamation as affected by corn stalk application and incubation: a laboratory study. Pedosphere 19:465–475
Singh K, Pandey VC, Singh B, Singh RR (2012) Ecological restoration of degraded sodic lands through afforestation and cropping. Ecol Eng 43:70–80
Singh K (2016) Microbial and enzyme activities of saline and sodic soils. Land Degrad Dev 27:706–718
Khatri-Chhetri A, Aggarwal PK, Joshi PK, Vyas S (2017) Farmers’ prioritization of climate-smart agriculture (CSA) technologies. Agri system 151:184–191
FAO (2014) Save and grow in practices: maize rice and wheat. http://www.fao.org/ag/save-and-grow/MRW/en/1/index.html
Latef AA, Tran LP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front Plant Sci 7:243
Salazar S, Sánchez LE, Alvarez J et al (2011) Correlation among soil enzyme activities under different forest system management practices. Ecol Eng 37:1123–1131
Islam F, Yasmeen T, Ari MS et al (2016) Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul 80:23–36
Dixit VK, Misra S, Mishra SK et al (2020) Characterization of plant growth-promoting alkalotolerant Alcaligenes and Bacillus strains for mitigating the alkaline stress in Zea mays. Antonie Van Leeuwenhoek 9:1–7
Mbarki S, Cerdà A, Brestic A et al (2017) Vineyard compost supplemented with Trichoderma Harzianum T78 improve saline soil quality. Land Degrad Dev 28:1028–1037
Mishra SK, Khan MH, Misra S et al (2017) Characterization of Pseudomonas spp. and Ochrobactrum sp. isolated from volcanic soil. Antonie Van Leeuwenhoek 10:253–270
Mishra SK, Khan MH, Misra S et al (2020) Drought tolerant Ochrobactrum sp. inoculation performs multiple roles in maintaining the homeostasis in Zea mays L. subjected to deficit water stress. Plant Physiol Biochem 150:1–4
Nautiyal CS (1997) A method for selection and characterization of rhizosphere-competent bacteria of chickpea. Current Microbiol 34:12–17
Khan N, Mishra A, Chauhan PS et al (2012) Paenibacillus lentimorbus enhances growth of chickpea (Cicer arietinum L.) in chromium-amended soil. Antonie Van Leeuwenhoek 1:453–459
Srivastava S, Chaudhry V, Mishra A et al (2012) Gene expression profiling through microarray analysis in Arabidopsis thaliana colonized by Pseudomonas putida MTCC5279, a plant growth promoting rhizobacterium. Plant Signal Behav 7:235–245
Nautiyal CS, Srivastava S, Chauhan PS et al (2013) Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem 66:1–9
Singh A, Chauhan PS (2022) N-acyl homoserine lactone mediated quorum sensing exhibiting plant growth-promoting and abiotic stress tolerant bacteria demonstrates drought stress amelioration. J Pure App Microbiol 16(1):669–684
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Joshi H, Mishra SK, Prasad V, Chauhan PS (2023) Bacillus amyloliquefaciens modulate sugar metabolism to mitigate arsenic toxicity in Oryza sativa L. var Saryu-52. Chemosphere 137070
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annal Biochem 72:248–254
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Hemeda HK, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, London
Tabatabai TA (1982) Soil enzymes. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis: II. Chemical and microbiological properties, 2nd edn. Am Soc Agron, Agronomy 10, Madison, Wisconsin, pp 903–947
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Ladd JN, Butler JHA (1972) Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol Biochem 4:19–30
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, et al. (eds) Methods of soil analysis: microbiological and biochemical properties. Part 2. SSSA book series no. 5. SSSA, Madison, pp 775–883
Muyzer G, De Waal EC, Uitterlinden A (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Paul D, Lade H (2014) Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron Sustain Dev 34:737–752
Vivekanandan M, Karthik R, Leela, (2015) Improvement of crop productivity in saline soils through application of saline-tolerant rhizosphere bacteria-Current perspective. Int J Curr Adv Res 3:1273–1283
İpek M, Eşitken A (2017) The actions of PGPR on micronutrient availability in soil and plant under calcareous soil conditions: an evaluation over Fe nutrition. In: Plant-microbe interactions in agro-ecological perspectives. Springer, Berlin, pp 81–100
Misra S, Dixit VK, Khan MH et al (2017) Exploitation of agro-climatic environment for selection of 1- aminocyclopropane-1-carboxylic acid (ACC) deaminase producing salt tolerant indigenous plant growth promoting rhizobacteria. Microbiol Res 205:25–34
Souza RD, Ambrosini A, Passaglia LMP (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38:401–419
Heydarian Z, Yu M, Gruber M, Glick BR et al (2016) Inoculation of soil with plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression of the corresponding acdS gene in transgenic plants increases salinity tolerance in Camelina sativa. Front Microbiol 7:1966
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica. https://doi.org/10.6064/2012/963401
Paganin P, Isca C, Tasso F et al (2023) A bacterial formula with native strains as alternative to chemical fertiliser for tomato crop. Plant Growth Regul. https://doi.org/10.1007/s10725-023-00993-3
Damodaran T, Mishra VK, Jha SK et al (2019) Identification of Rhizosphere bacterial diversity with promising salt tolerance, PGP traits and their exploitation for seed germination enhancement in sodic soil. Agric Res 8:36–43
Singh RP, Jha PN (2016) The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS One 11:e0155026
Tiwari S, Lata C, Chauhan PS, Nautiyal CS (2016) Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem 99:108–117
Housh AB, Noel R, Powell A et al (2023) Studies using mutant strains of Azospirillum brasilense reveal that atmospheric nitrogen fixation and auxin production are light dependent processes. Microorganisms 11(7):1727
Choudhary DK, Kasotia A, Jain S, Vaishnav A et al (2016) Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J Plant Growth Regul 35:276–300
Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res Int 2016:6284547
Ravi B, Foyer CH, Pandey GK (2023) The integration of reactive oxygen species (ROS) and calcium signalling in abiotic stress responses. Plant, Cell Environ 46:1985–2006
Kumar M, Mishra S, Dixit V et al (2016) Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal Behav 11:e1071004
Naveed M, Mitter B, Reichenauer TG et al (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 1:30–39
Xiu-Mei LIU, Qi LI, Wen-Ju L, Yong J (2008) Distribution of soil enzyme activities and microbial biomass along a latitudinal gradient in farmlands of Songliao Plain, Northeast China. Pedosphere 18:431–440
Nayak T (2017) Effect of long-term fertilization and manuring on soil quality index and carbon stock under rice-wheat cropping system in vertisols. PhD diss., Indira Gandhi Krishi Vishwavidhyalaya, Raipur http://krishikosh.egranth.ac.in/handle/1/5810025198
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci 26:1–20
Çam S, Küçük Ç, Almaca A (2023) Bacillus strains exhibit various plant growth promoting traits and their biofilm-forming capability correlates to their salt stress alleviation effect on maize seedlings. J Biotechnol 369:35–42
Bhat MA, Mishra AK, Jan S et al (2023) Plant growth promoting rhizobacteria in plant health: a perspective study of the underground interaction. Plants 12(3):629
Oburger E, Gruber B, Schindlegger Y et al (2014) Root exudation of Phyto siderophores from soil grown wheat. New Phytol 203:1161–1174
Keiluweit M, Bougoure JJ, Nico PS et al (2015) Mineral protection of soil carbon counteracted by root exudates. Nat Clim Chang 5:588–595
Shanmugam SG, Kingery WL (2018) Changes in soil microbial community structure in relation to plant succession and soil properties during 4000 years of pedogenesis. Eur J Soil Biol 88:80–88
Chandra LR, Gupta S, Pande V, Singh N (2016) Impact of forest vegetation on soil characteristics: a correlation between soil biological and physico-chemical properties. 3 Biotech 6:188
Valarini PJ, Alvarez MCD, Gasco JM et al (2003) Assessment of soil properties by organic matter and EM-microorganism incorporation. Revista Brasileira De Ciencia Do Solo 27:519–525
Wu J, Yang YS, Lin J (2005) Advanced tertiary treatment of municipal wastewater using raw and modified diatomite. J Hazard Mater 127:196–203
Schmidt MWI, Torn MS, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Sinsabaugh RL, Lauber CL, Weintraub MN et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Nannipieri P, Ceccanti B, Cervelli S, Matarese E (1980) Extraction of phosphatase, urease, proteases, organic carbon, and nitrogen from soil. Soil Sci Society America J 44:1011–1016
Nannipieri P, Ascher J, Ceccherini M et al (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
Ellis RJ, Morgan P, Weightman AJ, Fry JC (2003) Cultivation-dependent and-independent approaches for determining bacterial diversity in heavy-metal-contaminated soil. Appl Environ Microbiol 69:3223–3230
Chaudhry V, Rehman A, Mishra A et al (2012) Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb Ecol 64:450–460
Misra S, Dixit VK, Mishra SK, Chauhan PS (2019) Demonstrating the potential of abiotic stress-tolerant Jeotgalicoccus huakuii NBRI 13E for plant growth promotion and salt stress amelioration. Ann Microbiol 69:419–434
Gupta S, Kaushal R, Spehia RS et al (2017) Productivity of capsicum influenced by conjoint application of isolated indigenous PGPR and chemical fertilizers. J Plant Nutr 40:921–927
Sood G, Kaushal R, Chauhan A, Gupta S (2018) Effect of conjoint application of indigenous PGPR and chemical fertilizers on productivity of maize (Zea mays L.) under mid hills of Himachal Pradesh. J Plant Nutr 41:297–303
Acknowledgements
The authors acknowledge the Director, CSIR-National Botanical Research Institute, for providing facilities and support during the study.
Funding
The authors acknowledge the Director, CSIR-National Botanical Research Institute for providing facilities and support during the study. This work is supported by in-house project (OLP116) funded by the Council of Scientific and Industrial Research, New Delhi, India.
Author information
Authors and Affiliations
Contributions
PSC conceived and coordinated the research. SKM, SM, VKD, and SK conducted the experiments and analyzed the data. PSC, SKM, and SM wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, S.K., Misra, S., Dixit, V.K. et al. Ochrobactrum sp. NBRISH6 Inoculation Enhances Zea mays Productivity, Mitigating Soil Alkalinity and Plant Immune Response. Curr Microbiol 80, 328 (2023). https://doi.org/10.1007/s00284-023-03441-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03441-7