Abstract
A study was undertaken to determine the effects of a strain of Arthrobacter sp., a Plant Growth-Promoting Bacteria (PGPB), on plant phenology and qualitative composition of Opuntia ficus-indica (L.) Mill. fruits and cladodes. The strain was inoculated in soil, and its effects on cactus pear plants were detected and compared to nontreated plants. Compared to the latter, the treatment with bacteria promoted an earlier plant sprouting (2 months before the control) and fruitification, ameliorating fruit quality (i.e., improved fresh and dry weight: + 24% and + 26%, respectively, increased total solid content by 30% and polyphenols concentrations by 22%). The quality and quantity of monosaccharides of cladodes were also increased by Arthrobacter sp. with a positive effect on their nutraceutical value. In summer, the mean values of xylose, arabinose, and mannose were significantly higher in treated compared to not treated plants (+ 3.54; + 7.04; + 4.76 mg/kg d.w. respectively). A similar trend was observed in autumn, when the cladodes of inoculated plants had higher contents, i.e., 33% xylose, 65% arabinose, and 40% mannose, respect to the controls. In conclusion, Arthrobacter sp. plays a role in the improvement of nutritional and nutraceutical properties of cactus pear plants due to its capabilities to promote plant growth. Therefore, these results open new perspectives in PGPB application in the agro-farming system as alternative strategy to improve cactus pear growth, yield, and cladodes quality, being the latter the main by-product to be utilized for additional industrial uses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Farm to Fork Strategy is the heart of the European Green Deal aiming to make food systems fair, healthy, and environmentally friendly. Therefore, following EU strategy, the current agriculture is implemented by more sustainable cultivation practices. The use of Plant Growth-Promoting Bacteria (PGPB) in many crop species has proven to be a promising practice in sustainable cultivation due to their positive effects on plant growth, yield quantity and quality, and on better adaptability to both biotic and abiotic stresses [1,2,3]. These microorganisms, applied to the soil can act on the plant either directly by producing (i.e., synthesis of plant growth regulators hormones, volatile organic compounds, trehalose, exopolysaccharides) and/or regulating the metabolism of different molecules (e.g., by ACC deaminase activity, or indirectly via pathogen antagonism or induced systemic resistance [1, 4, 5]). Several studies have shown the positive effect of PGPB on different crops [2, 6,7,8,9], thus, implementing the interest towards such promising bacterial strains and their possible application in agronomic field. Among plant growth-promoting species, Arthrobacter sp. has shown a high adaptability to interact with different plant species [2, 10,11,12,13]. The strain was originally isolated from the leaf cavities of the fern Azolla filiculoides Lam., where the bacteria are also localized within the sporocarps [12,13,14]. Further characterization of the bacteria included the synthesis of polysaccharides [14] and IAA [15], halotolerance and lack of ACC deaminase activity [2, 10, 16], and the ability to help plants in counteracting salt stress [2, 10]. Actinobacteria have been also isolated from Opuntia ficus-indica, the molecular phylogeny of the isolated strains revealed the components of the distinct clades of Streptomyces spp. quite away from other actinobacterial genera [17, 18]; these bacteria may play a role in drought-tolerant species with potential amelioration of plant growth, especially under abiotic stress conditions. Basing on the under-exploited plant-bacteria interactions in cactus species, and the promising results obtained in other crops, the aim of this work was to explore the potential compatibility of the use of Arthrobacter sp. in drought-tolerant crop. Opuntia ficus-indica (L.) Mill. (OFI), which belongs to the family Cactaceae, is an important crop in agricultural economies, mainly in arid and semi-arid parts of the world, due to its crassulacean acid metabolism (CAM) and its great efficiency in water utilization and conservation [19, 20]. Its fruits are very popular and appreciated in many countries for their nutraceutical and nutritional properties with an increasing industrial interest on cladodes as raw materials for feed, food, cosmetic, pharmaceutical applications [21,22,23,24]. The great amount of mucilage, mainly composed by high molecular weight polysaccharides (arabinose, xylose, rhamnose), makes cladodes a remarkable source for hydrocolloid market addressed to functional ingredients which is expected to reach 13.36 billion USD by 2026 [24]. Italy is the most important supplier of cactus pear in Europe with the cultivation mainly located in Sicilian specialized orchards (90% of the national production) [25]. Although this crop is considered a rustic species, the implementation of modern cultivation practices enhances crop productivity, quality, and sustainability; especially in organic agriculture system, the request for plant bio-stimulants, biological fertilizer, or biofertilizer is increasing in the last years. Therefore, this study aims to contribute to the discussion on the effectiveness of using PGPB in agriculture. More specifically, the aim of this work lays on Arthrobacter sp. soil inoculation and its effects on cactus pear plant growth, yield, and cladodes quality. To this end, the study of biometric parameters, i.e., cladodes and fruits (weight sizes), as well as biochemical characteristics (polyphenols, antioxidant activity, polysaccharides content) were quantified as measures of the plant fitness and compared with those obtained from not inoculated plants. The use of alginate beads as bacteria inoculum tool has been also considered.
Experimental Setup
Bacteria Inocula Preparation
Arthrobacter sp. strain CD belongs to the collection of the Laboratory of Botany and Phytotechnologies, Department of Biology, University of Rome Tor Vergata. The strain, isolated from Azolla leaf cavities, was identified according to standard procedure and compared with Arthrobacter globiformis type strain ATCC 8010 of Pasteur Institute [26].
The strain was stored in glycerol solution at − 80 °C. The bacteria were grown in tryptic soy broth (TSB) medium (Sigma-Aldrich) at 30 °C and refreshed twice a week with fresh medium.
To encapsulate, formulate, and apply Arthrobacter as bioinoculant, alginate beads were considered [16, 27]. The bacterial culture (OD600 = 1.3) was diluted (1:2 v/v) in a sterile solution of Na+-alginate (6% w/v) to a final concentration of 3% alginate. The addition of sterile CaCl2 solution (0.2 M) allowed the formation of beads [16]. The beads were washed 3 times with sterile milli-Q water and stored at room temperature in sterile conditions.
The inoculum was prepared by re-suspending the beads in medium as follows. Fifty-six beads were divided into 7 tubes of 50 mL volume. Twenty mL of minimal culture medium (saline solution (0.9% NaCl) added with 2.5 g/L glucose) was added to the tubes. After 48 h, the cultures (OD 600 nm = 7 × 106 CFU) were ready to be inoculated near the roots of acclimated plants.
Plant Materials and Phenological Evaluations
Cladodes were collected in specialized cactus pear orchard of the Gaetano Spitale farm located in Mazzarino (CL) Italy, 37°16′14.9"N 14°20′17.4"E. The cladodes (2 years old) were immediately planted in pots, containing the same soil of the field (sand 724 g/kg; silt 70 g/kg; clay 206 g/kg; pH 7; electrical conductivity 0.7 dS/m; total porosity 80% (v/v). The temperature at the time of transplanting was 23 °C and RH 68%. The plants were acclimated for 5 months, and then inoculated as reported above. A complete randomized block design was applied in the experiments: i.e., the pots were randomly divided in two sets, a first set was inoculated with bacteria, and the other noninoculated was used as control. Each month the growth of the plants was evaluated by (1) time of sprouting, (2) number and size of new cladodes, (3) flowering time, and (4) number of fruits per plant. Phenological observations were conducted for 2 years.
Samples of cladodes (in summer and autumn) and fruits (in autumn) were collected from inoculated and not inoculated plants. All samples were oven dried at 60 °C for 72 h and maintained in dry conditions until analyses.
Evaluation of Fruit Characteristics
Fresh and dry weights were determined on raw or dried cactus per fruits; total soluble solids (% Brix) were evaluated on juices, obtained from fruits peeled and homogenized by blade homogenizer. The juices filtered and diluted (1:10 v/v) in distilled water were analyzing using HI96801 refractometer (Hanna instrument).
Extraction and Quantification of Total Phenolic Compounds
Total polyphenols and antioxidant activity were evaluated on dried cladodes and fruits. We used the Folin–Ciocâlteu method as reported by Procacci et al. [22] with slight modifications. Briefly, an aliquot of 200 µL of standard solution, extract of sample (40 mg in 1 mL ethanol 80% at 30 °C for 30 min), or 80% methanol (control) was mixed with 1.5 mL of Folin–Ciocâlteu reagent (Sigma-Aldrich), which was previously diluted with double-distilled water (1:10). The mixture was allowed to stand for 5 min, and then 1.5 mL of sodium carbonate (60 g/L) was added to the mixture. Finally, the mixture was allowed to stand in the dark for about 30 min and the absorbance (Abs) was measured at 725 nm against a blank solution using a UV/Visible spectrophotometer (Lambda 2—Perkin Elmer). All measurements were carried out in triplicate, and results were expressed as milligram of gallic acid eq/g dry weight (mg Gallic Acid eq/g d.w.). A calibration curve was performed with gallic acid as standard (y = 0.0031x − 0.0338, R2 = 0.9981).
Flavonoids were estimated following this procedure [28]: 200 mg of dry material was homogenized in liquid nitrogen and re-suspended in 5 mL ethanol (95% v/v), incubated overnight at 4 °C, and then centrifuged at 8000 g for 15 min. The supernatants were stored at − 20 °C until analysis. The sample absorbance was measured at 415 nm. To calculate the concentration of flavonoids, a calibration curve was performed using quercetin (Sigma) as standard at different concentrations (10 μg/mL, 20 μg/mL, 40 μg/mL, and 80 μg/mL; y = 0.0081x − 0.0321, R2 = 0.999). Flavonoids are expressed as μg quercetin eq/g d.w.
Antioxidant Activity
Antioxidant capacity was determined by DPPH method [22]. Briefly, 40 mg of each sample was extracted with 1 mL of 70% methanol at 30 °C × 30 min and filtered at 0.2 µm. 100 µL of each extract was, thus, added to 3.9 mL of a DPPH radical solution 0.06 mM, mixed in a Vortex and kept in the dark conditions × 60 min before reading the absorbance at 517 nm (spectrophotometer Varian CARY 50 Scan). The antioxidant activity was obtained from the decrease in absorbance of DPPH (% inhibition), from which, by interpolation, the results were expressed as mg of Trolox equivalent per gram of dry weight (µmol TE g/d.w.). The percentage of inhibition was obtained by interpolation of a calibration curve (y = 0.1164x + 5.0225, R2 = 0.9999) (Abs vs. µM Trolox)
A0 = absorbance of control sample (t = 0 h) and As = absorbance of a tested sample at the end of the reaction.
Cladodes Sugar Composition
The sugars were extracted from 10 g of the finely ground-dried cladode sample with a mixture acetonitrile/water 60:40 (v/v) under constant stirring for 3 h. Monosaccharide’s composition was evaluated by HPLC using specific chromatographic column (Amino Carbohydrate Waters, Millipore); the revelation was carried out by means of a refractive index detector. The HPLC pump has been set up to flow approximately at 1.0 mL/min, and mobile phase was acetonitrile/water 75:25 (v/v). The content of the individual monosaccharides, expressed as a percentage, was calculated according to the following formula:
Ac = area of the sample peak, AS = area of the peak of the standard, Ms = quantity of standard injected expressed in g, and Mc = quantity of sample injected expressed in g [29].
Statistical Analysis
The experiments were carried out in three replications. Data of biological feasibility studies were subjected to one-way Analysis of Variance (ANOVA) by program Jamovi software version 2.3.1. Data means were compared with Fisher’s protected Least-Significant Difference (LSD) test at probability level of 0.05. Differences at the P ≤ 0.05 levels were considered significant. When comparing inoculated groups with noninoculated ones, the significance was reported as ***P < 0.001; **P < 0.01; *P < 0.05.
Results
Effect of Bacteria on Plant Phenological Phases
Inoculated plants started to produce new cladodes 3–4 months after the treatment, a delay of 1 or 2 months in sprouting was observed in the controls both in the first and second years of experiment (Fig. 1). Significant differences were also found both in the number and size of newly differentiated cladodes. As reported in Fig. 1, inoculated plants formed 3–4 cladodes/plant from February–March to May with the untreated plants 1–2 cladodes/plant from April to May (data confirmed in the 2 years of observation). Furthermore, the surface area of cladodes after 1 month from differentiation was greater in treated plants (+ 56.03% ± 1.31 and + 57.53% ± 1.28 in the first and the second years, respectively) when compared to the newly pad average area of the control (see Table A_Online Resource 1).
Number of new cladodes developed in plants treated with Arthrobacter sp. compared to the control. Inoculated plants anticipated sprouting of 1 or 2 months during the first and the second years of evaluation, respectively. Data collected from October to June in the 2 years of the experiment are expressed as mean ± SD of three replications of five plants each. Significant differences between not inoculated and inoculated plants by ANOVA test determined by ANOVA test, are reported as ***P < 0.001
Therefore, the time of flowering was anticipated of about 2 months in inoculated plants; consequently, the inoculated plants prolonged fruiting season providing a second harvest at the beginning of autumn season (average 6.0 ± 0.5 fruits/plant), when the controls just developed the first fruits (not more than 2–3 for year).
Effect of Bacteria on Physio-biochemical Parameters of Fruits
The inoculation with Arthrobacter sp. positively influenced the fruit weight with an increase equal to 31% in fresh fruit weight, which led to a higher dried weight (+ 26%) and total soluble solids (+ 3.87% °Brix) when compared to untreated plants (Table 1).
Moreover, Arthrobacter sp. positively affected the total polyphenol (TP) content of the fruits collected in August. As reported in Table 1, the mean value of TP was significantly higher in treated (15.6 mg Gallic Acid eq/g d.w.) compared to nontreated plants (13.94 mg Gallic Acid eq/g d.w.)
Non significant effect was observed for total flavonoids content of samples (values ranged from 5.94 µg quercetin eq./g d.w. in nontreated plants to 7.65 µg quercetin eq./g d.w. in treated ones).
Data, from DPPH assays on fruits, revealed the positive influence of bacteria inoculation on antioxidant activity. The samples of treated plants showed an increment of bioactive molecules activity if compared to untreated ones (15.6 µmol Trolox eq./g d.w., 10.9 µmol Trolox eq./g d.w., respectively).
Effect of Bacteria on OFI Cladodes Nutraceutical Composition
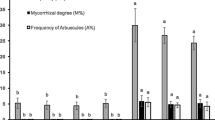
The presence of bacteria significantly enhanced the amounts of xylose, mannose, and arabinose in cladodes (Fig. 2). In summer, the mean values of these monosaccharides were significantly higher in treated compared to untreated plants (29.11 mg xylose/kg d.w. vs. 25.57 mg xylose/kg d.w.; 31.01 mg arabinose/kg d.w. vs. 24.01 mg arabinose/kg d.w. and 22.20 mg mannose/kg d.w. vs. 17.44 mg mannose/kg d.w.). A similar trend was observed in autumn (40.86 mg xylose/kg d.w. vs. 60.93 mg xylose/kg d.w.; 1.06 mg arabinose/kg d.w. vs. 3.06 mg arabinose/kg d.w. and 22.60 mg mannose/kg d.w. vs. 13.46 mg mannose/kg d.w.)
Effect of Arthrobacter sp. inoculation on monosaccharide composition of cladodes sampled in summer and autumn (a = Xylose; b = Mannose; c = Arabinose). The presence of bacteria significantly enhanced the amounts of xylose, mannose and arabinose in cladodes when compared to those of noninoculated plants both in summer and autumn. Data, determined by HPLC analysis in triplicate, are expressed as mean ± SD. Significant differences between noninoculated and inoculated plants determined by ANOVA test are reported as ***P < 0.001
Results of the Folin–Ciocâlteu assay (Fig. 3) performed on cladode samples, indicated a minor content of total polyphenols in plants inoculated with Arthrobacter sp. These differences were significantly marked in cladodes collected in June (14.5 mg Gallic Acid eq/g d.w. in inoculated vs. 23.54 mg Gallic Acid eq/g d.w. in noninoculated) respect to those sampled in autumn (17.23 mg Gallic Acid eq/g d.w. in inoculated and 18.25 mg Gallic Acid eq/g d.w. in noninoculated).
Total phenolic content in cladodes sampled in summer and autumn from plants inoculated and noninoculated with Arthrobacter sp. Results of the Folin–Ciocâlteu assay, indicated a minor content of total polyphenols in plants inoculated with bacteria; these differences were significantly marked in cladodes collected in June respect to those sampled in autumn. Data are expressed as mean ± SD of three replications and three spectrophotometric readings each one. Significant differences by ANOVA test are reported as ***P < 0.001 and *P < 0.05
Among the total polyphenols, the content of flavonoids, which are a characteristic component of cactus pear, was significantly lower in samples from plants treated with bacteria (51.54 µg quercetin eq/g d.w.) respect to those untreated (73.87 µg quercetin eq/g d.w.) (Fig. 4). No differences were found in the same samples collected in autumn, which were characterized by a minor content of flavonoids (37.79 µg quercetin eq/g d.w. and 38.10 µg quercetin eq/g d.w. respectively) if compared to those of summer season.
Flavonoids content in cladodes sampled in summer and autumn from plants inoculated and noninoculated with Arthrobacter sp. The content of flavonoids was significantly lower in samples from plants treated with bacteria respect to those untreated. No differences were found in the same samples collected in autumn, which were characterized by a minor content of flavonoids if compared to those of summer season. Data are expressed as mean ± SD of three replications and three spectrophotometric readings each one. Significant differences by ANOVA test are reported as **P < 0.01
The antioxidant activity performed by DPPH method is reported in Fig. 5. Data showed that the antioxidant activity in cladodes was increased in inoculated samples, collected in both summer (6.96 µmol Trolox eq/g d.w.) and autumn (6.75 µmol Trolox eq/g d.w.) when compared to the controls (5.23 µmol Trolox eq/g d.w. and 4.53 µmol Trolox eq/g d.w.), respectively.
Antioxidant activity in cladodes sampled in summer and autumn from plants inoculated and not inoculated with Arthrobacter sp. The antioxidant activity performed by DPPH method showed that the antioxidant activity in cladodes was increased in inoculated samples, collected in both summer and autumn when compared to the controls. Data are expressed as mean ± SD of three replications and three spectrophotometric readings each one. Significant differences by ANOVA test are reported as ***P < 0.001
Discussion
Under the perspective of climate changes, increasing of soil salinization and aridity, the possibility of using PGPB can represent a useful approach to improve plant tolerance to abiotic stresses. Among these bacteria, Arthrobacter sp. has been proved to be an excellent microbial inoculum in rapeseed exposed to salinity [2, 10, 11]. Besides of living in the symbiotic association of Azolla-Anabaena [15], this species is also widespread in soils [30, 31]. Moreover, the possibility to use bioencapsulated microorganisms as inoculants represents a further benefit to a wider utilization of bacteria inocula [27].
Since Arthrobacter sp. can produce IAA [15], thus, influencing cactus pear growth anticipated the yield, and improved some qualitative characteristics of fruits and cladodes. In fact, inoculated plants differentiated more cladodes of bigger sizes, and anticipated the flowering (1–2 months before the control), with an enhancement of the number of fruits per plants (see Figure A_Online Resource 2). Based on these results, the opportunity to lengthen the fruit picking period provides positive impact on the fruit supply and commercialization, which is particularly interesting for the national and international markets. In similar research, Morais et al. [7] recently reported that PGPB inoculation significantly accelerated crop maturation in strawberry. The same authors proved also the inoculation with a strain of Pedobacter, led to an enhancement in the dimensions of fruits, especially fruit length and shape, as well as in the total soluble solids content (°Brix). A part of strawberry, comparable results were reported for other crops, i.e., raspberry, tomato, sugar, beet, and barley [8, 32,33,34,35,36]. Our work, in agreement with these authors, demonstrated that PGPB treatments led to an increase equal to 21% of fruit dry weight per plant compared to the control with a significant increase in the number of fruits/cladodes. Furthermore, the bacteria inoculation significantly increased the °Brix in OFI fruits with a positive impact on fruit sweetness. The benefits of Arthrobacter sp. in boosting plant growth and fructification were confirmed in the second year of growth.
In general, the application of bio-stimulants may increase the synthesis of bioactive compounds in plants. Inoculation with Arthrobacter sp. led to a significant increase in total polyphenols, but not in flavonoid content or antioxidant activity of fruits. Similar results were shown by Esitken et al. [32] in strawberry nutritional contents and by Katsenios et al. [8] who showed an increase of total soluble solids, lycopene and total carotenoids amount which improved the tomato industrial quality. Our preliminary analysis, performed by HPLC on cactus pear fruits juices, also revealed the effect of Arthrobacter treatment on bioactive compound profiles. By analyzing the signal intensities detected in the samples at 280 nm, 320 nm, and 360 nm, a significant higher signal intensity was detected at 320 nm in samples from inoculated plants (see Table B_Online Resource 3). These results indicated a prevalent presence of cinnamic and hydroxycinnamic acids, chlorogenic acid, and other caffeoylquinic acids characterized by maximum absorption around this wavelength. Furthermore, fruits from plants treated with bacteria showed higher level of benzoic acids, hydroxybenzoic acids, catechins, and flavan-3-oils (maximum absorption at 280 nm) if compared to the control.
Concerning the cladodes, Ribeiro et al. [37] showed a seasonal variation on sugar amount; moreover, Da Silveira Agostini-Costa [38] reported carbohydrate, ash, and polyphenol contents were influenced by both environment and ripening stage. More recently, Messina et al. [39] found a significant and continuous increase, from March to October, of carbohydrates content in cladodes mucilage, which was negatively correlated (− 0.99, P < 0.01) with the water content. Our results demonstrated that samples from cladodes contained a good quantity of neutral and acid sugars in both summer and autumn seasons. In particular, samples collected in the summer presented a higher content of carbohydrates especially arabinose and mannose. It is, therefore, conceivable that the changes in the carbohydrate compositions, observed in cladodes, are related to a more efficient use of water and energy status of plants. Alterations in carbohydrate utilization and metabolism were also discussed by different authors in the case of abiotic stresses, such as nitrogen [40] or drought stress [41].
Interestingly, Arthrobacter enhanced the quantity of monosaccharides in cladodes, such as xylose, mannose, and arabinose when compared to samples from not inoculated plants. The latter showed a high xylose content in the autumn season, when mannose and arabinose decreased. Similar trend was observed in inoculated plants for xylose and arabinose (which were always significantly higher than control), while higher concentration of mannose was detected in treated samples in both summer and autumn. This monosaccharide, which is an epimer of the d-glucose, when associated with galactose (mannogalactans) forms the basis of complex polysaccharides, such as the mucilage with a key function in the plant hydration. These changes may be the results of the effects of PGPB, reported as enhancers of plant–water relations, plant nutritional status, and plant response to stresses, either in the rhizosphere, or as endophytes or in bio-primed plants [2, 10, 42, 43].
Nevertheless, this stimulating action, demonstrated for the first time in Opuntia, offers an interesting perspective to produce bio-products, such as flour with high monosaccharide contents suitable for nutraceutical purposes.
On the other hand, amounts of phenolics and flavonoids were significantly higher in OFI cladodes of not inoculated plants in both seasons. In particular, the content of flavonoids, which are the main fractions of Opuntia spp., showed a significant increment in summer cladodes samples of untreated plants (see also Table B_online Resources 3). Literature reports suggest that polyphenolic compounds and flavonoids act as reducing agents by being either electron donors or enzyme co-factor [44, 45]. The synthesis of these compounds can usually be enhanced in organs under stress conditions. Therefore, novel-microbe-assisted technologies can assist plants in withstanding stress conditions enhancing their tolerance and productivity. It is to note that increased temperatures decrease crop productivity by affecting biochemical, physiological, molecular, and morphological plant traits. Khan et al. [46] reported that the inoculation with the thermo-tolerant Bacillus cereus improved the biomass, the content, and fluorescence of chlorophylls, and antioxidant capability of soybean plants under heat stress. From this point of view, the application of PGP microbes ameliorated stress response in many crops, such as wheat, rice, maize, and rapeseed primarily by increasing nutrient availability [2, 11, 47]. In potato genotypes tolerant to heat stress, photosynthetic rates were unaffected or increased slightly at the higher temperature, while heat stress increased accumulation of foliar sucrose and decreased starch accumulation in mature leaves [48]. A similar mechanism could be hypothesized for cactus pear considering the significant accumulation of monosaccharaides in cladodes.
The application of PGPB offers an ecofriendly approach for improving crop production and counteracting the negative effects of abiotic stress [1, 47]. The soil application of Arthrobacter sp. on Opuntia ficus-indica plants improved the antioxidant activity of cladodes extracts with statistically significant differences compared to the control. The increased antioxidant activity may be attributed to the presence of antioxidant compounds, such as ascorbate (vitamin C), tocopherol (vitamin E), and flavonoids, playing important role in non-enzymatic antioxidant activity of Opuntia that has the capability to improve plant physiological status in response to adverse environmental factors. Further work is in progress to determine the presence of vitamins and the possible antioxidant activity by reducing groups of monosaccharides [49]
From these results, it can be reported that the application of Arthrobacter sp. on OFI is able to mitigate the negative effect on growth due to seasonal conditions, such as high temperatures and aridity in summer, impacting also on phenological stage, like fruit differentiation in autumn.
The results of this experiment confirmed the possibility of a wide application of Arthrobacter species as inoculum in different crops [2, 10, 18]. So far, this is the first report on the positive effects of this bacteria species on fruits ripening and quality of OFI.
Conclusions
Due to its ‘multi-functionality,’ OFI is indicated as one of the species of the future, and our results may contribute to disclose its potentialities in the contest of bioeconomy and circular economy. Since natural resources and cultural practices are crucial in defining the quality of cladodes when addressed to food/nutraceutical applications, the inoculation of OFI with Arthrobacter sp. may be foreseen to provide a better plant growth under environmental stress conditions, or as soil fertilizer, but also to improve the synthesis of natural products, used for therapeutic applications. Further studies would be aimed to better understand the connecting pathways involved in OFI-Arthrobacter interaction in order to assess bacteria contribution to cactus pear metabolism and to bridge the gap in the use of Arthrobacter sp. from laboratory to field scale.
References
Forni C, Duca D, Glick BR (2017) Mechanism of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 410:335–356. https://doi.org/10.1007/s11104-016-3007-x
Stassinos MP, Rossi M, Borromeo I, Capo C, Forni C (2022) Amelioration of salt stress tolerance in rapeseed (Brassica napus) cultivars by seed inoculation with Arthrobacter globiformis. Plant Biosyst 156:370–383. https://doi.org/10.1080/app11263504.2020.1857872
Ghazala I, Chiab N, Saidi MN, Radhia GB (2023) The plant growth-promoting bacteria strain Bacillus mojavensis I4 enhanced salt stress tolerance in durum wheat. Curr Microbiol 80:178. https://doi.org/10.1007/s00284-023-03288-y
Nieto KF, Frankenberger WT (1989) Biosynthesis of cytokinins by Azotobacter chroococcum. Soil Biol Biochem 21:967–972
Ruzzi M, Aroca R (2015) Plant growth-promoting Rhizobacteria act as biostimulants in Horticulture. Sci Hortic 196:124–134. https://www.mdpi.com/1420-3049/21/5/573
Vejan P, Abdullah R, Khadiran T, Ismail S, Boyce AN (2016) Role of plant growth-promoting rhizobacteria in agricultural sustainability—a review. Molecules 21:573. https://doi.org/10.3390/molecules21050573
Morais MC, Mucha A, Ferreira H, Gonçalves B, Bacelar E, Marques G (2019) Comparative study of plant growth-promoting bacteria on the physiology, growth and fruit quality of strawberry. J Sci Food Agric 99:5341–5349. https://doi.org/10.1002/jsfa.9773
Katsenios N, Andreou V, Sparangis P, Djordjevic N, Giannoglou M, Chanioti S, Stergiou P, Xanthou MZ, Kakabouki I, Vlachakis D, Djordjevic S, Katsaros G, Efthimiadou A (2021) Evaluation of plant growth promoting bacteria strains on growth, yield and quality of industrial tomato. Microorganisms 9:2099–2107. https://doi.org/10.3390/microorganisms9102099
Efthimiadou A, Katsenios N, Chanioti S, Giannoglou M, Djordjevic N, Katsaros G (2020) Effect of foliar and soil application of plant growth promoting bacteria on growth, physiology, yield and seed quality of maize under Mediterranean conditions. Sci Rep 10:21060. https://doi.org/10.1038/s41598-020-78034-6
Rossi M, Borromeo I, Capo C, Glick BR, Del Gallo M, Pietrini F, Forni C (2021) PGPB improve photosynthetic activity and tolerance to oxidative stress in Brassica napus grown on salinized soils. Appl Sci 11:442–447. https://doi.org/10.3390/app112311442
Heidary M, Golpayegani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agric Sci 11:57–61. https://doi.org/10.1016/j.jssas.2011.09.001
Grilli CM, Forni C, Castagnola M (1988) Bacteria in the Azolla-Anabaena association. Symbiosis 5:185–198. https://doi.org/10.1007/978-94-009-0889-5-10
Forni C, Grilli CM, Gentili S (1989) Bacteria in the Azolla-Anabaena symbiosis. In: Skinner FA, Boddey RM, Fendrik I (eds) ‘Nitrogen fixation with non-Legumes’ proceedings 4th international symposium on nitrogen-fixation with non-Legumes. Kluwer Academic Publishers, Dordrecht, pp 83–88
Forni C, Haegi A, Del Gallo M, Grilli Caiola M (1992) Production of polysaccharides by Arthrobacter globiformis associated with Anabaena azollae in Azolla leaf cavity. FEMS Microbiol 93:269–274. https://doi.org/10.1111/j.1574-6968.1992.tb05109.x
Forni C, Riov J, Grilli Caiola M, Tel-Or E (1992) Indole-3—acetic acid (IAA) production by Arthrobacter species isolated from Azolla. J Gen Microbiol 138:377–381. https://doi.org/10.1099/00221287-138-2-377
Rossi M (2022) Microbial tool to improve salt tolerance in plants. PhD THESIS in Evolutionary Biology and Ecology, University of Rome Tor Vergata, Rome, Italy. https://doi.org/10.38212/2224-6614.2748
Govindasamy V, Priya G, Ramesh SV, Sureshkumar V, Jagadish R, Minhas PS (2022) Characterization of root-endophytic actinobacteria from cactus (Opuntia ficus-indica) for plant growth promoting traits. Arch Microbiol 204:150. https://doi.org/10.1007/s00203-021-02671-2
Meng XX, Xu QZ, Xia Y, Chen C, Shen Z (2018) Complete genome sequence of Cd(II)-resistant Arthrobacter sp. PGP41, a plant growth-promoting bacterium with potential in microbe-assisted phytoremediation. Curr Microbiol 75:1231–1239. https://doi.org/10.1007/s00284-018-1515-z
Hernandez-Hernandez T, Brown JW, Schlumpberger B, Magall ELE, Beyond S (2014) Aridification: multiple explanations for the elevated diversification of cacti in the new world Succulent Biome. New Phytol 202:1382–1397. https://doi.org/10.1111/nph.12752
Nobel PS (2010) Desert wisdom/agaves and cacti: CO2, water, climate change. Universe Inc, Bloomington
Bacchetta L, Maccioni O, Martina V, Bojorquez-Quintal E, Persia F, Procacci S, Zaza F (2019) Quality by design approach to optimize cladodes soluble fiber processing extraction in Opuntia ficus-indica (L.) Miller. J Food Sci Technol 56:3627–3634. https://doi.org/10.1007/s13197-019-03794-7
Bojórqez-Quintal E, Bacchetta L, Ku-Gonzalez A, Procacci S, Ortega-Ordaz A, Sánchez-Rodríguez A, Cruz-Cárdenas C (2021) CAP. 4-Anatomía, fisiología y química de Opuntia-ficus-indica en un escenario de cambio climático. In: Alisi C, Insaurralde M (eds) Innovar desde la tradición. El collegio de Michoacán, ENEA, pp. 111–164. ISBN 978-607-544-159-7
Procacci S, Bojórquez-Quintal E, Platamone G, Maccioni O, Vecchio V, Morreale V, Alisi C, Balducchi R, Bacchetta L (2021) Opuntia ficus-indica pruning waste recycling: recovery and characterization of mucilage from cladodes. Nat Resour 12:91–107. https://doi.org/10.4236/nr.2021.124008
Hydrocolloids Market by Type, Source, Function, Application and Region—Global Forecast to 2026. Ed Markets and Markets (2021). ID: 3607249, p 271 https://www.reportlinker.com/p03607249/Hydrocolloids-Market-by-Type-Function-Source-Application-by-Region-Global-Forecast-to.html
Ochoa MJ, Barbera G (2017). History and economic and agro-ecological importance. In: Crop ecology, cultivation and uses of cactus pear. FAO and ICARDA (ed), pp 1–11. https://www.arc.agric.za/arc-ppri/weeds/PUBLICATIONS/Crop%20ecology,%20Cultivation%20and%20uses%20of%20Cactus%20pear.pdf
Forni C, Gentili S, Van Hove C, Grilli Caiola M (1990) Isolation and characterization of the bacteria living in the sporocarps of Azolla filiculoides Lam. Ann Microbiol Enzimol 40:235–243. https://doi.org/10.1111/j.1574-6968.1992.tb05109.x
Schoebitz M, López MD, Roldán A (2013) Bioencapsulation of microbial inoculants for better soil–plant fertilization. A review. Agron Sustain Dev 33:751–765. https://doi.org/10.1007/s13593-013-0142-0
Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:3–10. https://doi.org/10.38212/2224-6614.2748
Grossi M, Micco C, Chirico M, Arnaldi C (1985) Determination of sugars in soft flour products by GLC and HPLC. J Food Sci Nutr 14:429–434
Hagedorn C, Holt JG (1975) A nutritional and taxonomic survey of Arthrobacter soil isolates. Can J Microbiol 21:353–361. https://doi.org/10.1139/m75-050
Mongodin EF, Shapir N, Daugherty SC, DeBoy RT, Emerson JB (2006) Secrets of soil survival revealed by the genome sequence of Arthrobacter aurescens TC1. PLoS Genet 2:214–220. https://doi.org/10.1371/journal.pgen.0020214
Esitken A, Yildiz HE, Ercisli S, Donmez MF, Turan M, Gunes A (2010) Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci Hortic 124:62–66. https://doi.org/10.1016/j.scienta.2009.12.012
Orhan E, Esitken A, Ercisli S, Turan M, Sahin F (2006) Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents inorganically growing raspberry. Sci Hortic 111:38–43. https://doi.org/10.1016/j.scienta.2006.09.002
Cakmakci R, KantarF SF (2001) Effect of N2-fixing bacterial inoculations on yield of sugar beet and barley. J Plant Nutr Soil Sci 164:527–531. https://doi.org/10.1002/1522-2624(200110)164:5%3c527::AID-JPLN527%3e3.0.CO;2-1
Çakmakçı R, Mosber G, Milton AH et al (2020) The effect of auxin and auxin-producing bacteria on the growth, essential oil yield, and composition in medicinal and aromatic plants. Curr Microbiol 77:564–577. https://doi.org/10.1007/s00284-020-01917-4
Turan M, Ataoglu N, Sezen Y (2004) Effects of phosphorus solubilizing bacteria (Bacillus megaterium) on yield and phosphorus contents of tomato plant (Lycopersicon esculentum L.). In: Proceedings of the national fertilizer congress. Farming-industry-environment, pp 11–13 Tokat, Turkey. https://doi.org/10.1080/01904160902943197
Ribeiro EM, Silva MH, Lima Filho JL, Brito J, Silva M (2010) Study of carbohydrates present in the cladodes of Opuntia ficus-indica (fodder palm), according to age and season. Cienc Tecnol Aliment 30:933–939. https://doi.org/10.1590/S0101-20612010000400015
Da Silveira Agostini-Costa T (2022) Genetic and environment effects on bioactive compounds of Opuntia cacti. J Food Compos Anal 109:104514. https://doi.org/10.1016/J.Jfca.2022.104514
Messina CM, Arena R, Morghese M, Santulli A, Inglese P, Liguori G (2022) Seasonal characterization of nutritional, technological and antioxidant properties of Opuntia ficus-indica Mill. mucilage. Acta Hortic 1343:443–451. https://doi.org/10.17660/ActaHortic.2022.1343.56
Thomas W, Rufry JR, Steven CH, Richard JV (1988) Alterations in leaf carbohydrate metabolism in response to nitrogen stress. Plant Physiol 88:725–730. https://doi.org/10.1104/pp.88.3.725
Diniz AL, da Silva DIR, Lembke CG, Costa MDL, Ten-Caten F, Li F, Vilela RD, Menossi M, Ware D, Endres L, Souza GM (2020) Amino acid and carbohydrate metabolism are coordinated to maintain energetic balance during drought in sugarcane. Int J Mol Sci 30:9124–9129. https://doi.org/10.3390/iJms21239124
Chakraborti S, Bera K, Sadhukhan S, Dutta P (2022) Bio-priming of seeds: plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress. https://doi.org/10.1016/j.stress.2021.1000522
Mitra D, Mondal R, Khoshru B, Shadangi S, Das Mohapatra PK, Panneerselvam P (2021) Rhizobacteria mediated seed bio-priming triggers the resistance and plant growth for sustainable crop production. Microbiol Curr Res. https://doi.org/10.1016/j.crmicr.2021.100071
Pérez FJ, Villegas D, Mejia N (2002) Ascorbic acid and flavonoid–peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves. Phytochem Rev 60:573–580. https://doi.org/10.1016/50031-9422(02)00146-2
Yamasaki H, Sakihama Y, Ikehara N (1997) Flavonoid–peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115:1405–1412. https://doi.org/10.1104/pp.115.4.1405
Khan MA, Asaf S, Khan AL, Jan R, Kang SM, Kim KM, Lee IJ (2020) Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol 20:175. https://doi.org/10.1186/s12866-020-01822-7
Fiodor A, Singh S, Pranaw K (2021) The contrivance of plant growth promoting microbes to mitigate climate change impact in agriculture. Microorganisms 9:1841. https://doi.org/10.3390/microorganisms9091841
Lafta AM, Lorenzen JH (1995) Effect of high temperature on plant growth and carbohydrate metabolism in potato. Plant Physiol 109:637–643. https://doi.org/10.1104/depp109.2.637
Salehi E, Emam-Djomeh Z, Askari G, Fathi M (2019) Opuntia ficus indica fruit gum: extraction, characterization, antioxidant activity and functional properties. Carbohydr Polym 206:565–572. https://doi.org/10.1016/J.carbpol.2018.11.035
Funding
Open access funding provided by Ente per le Nuove Tecnologie, l'Energia e l'Ambiente within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors. The research is part of PhD Thesis.
Author information
Authors and Affiliations
Contributions
Conceptualization, GP, LB, and CF; Data curation, GP, IB, SP, and OM; Formal analysis, LB and GP; Methodology, LB, GP, SP, OM, IB, and MR; Resources, CF and LB; Software, GP; Supervision, CF: and LB; Validation, GP and MR; Visualization, CF and LB; Writing—original draft, GP, IB, MR, CF, and OM; Writing—review and editing, CF, LB, and SP. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Platamone, G., Procacci, S., Maccioni, O. et al. Arthrobacter sp. Inoculation Improves Cactus Pear Growth, Quality of Fruits, and Nutraceutical Properties of Cladodes. Curr Microbiol 80, 266 (2023). https://doi.org/10.1007/s00284-023-03368-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03368-z