Abstract
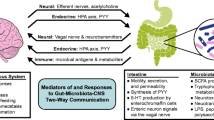
Multiple sclerosis (MS) is a chronic inflammatory disease characterized by central nervous system (CNS) lesions that can lead to severe neurological defects. Evidence is mounting that immune function is crucial in neuroinflammatory illnesses like MS. Through its impact on systemic immunological reactions, the large microbial population known as the gut microbiota has been linked to both human health and disease. The gut-brain axis (GBA) therefore encompasses neurological, immunological, and hormonal pathways, and microbiota can have a number of effects on the immune system, influencing MS. Recent research revealed a bidirectional relationship between metabolites originating from the gut microbiota, namely short-chain fatty acids (SCFAs). Intestinal epithelial cells are influenced by SCFAs, which also boosts the secretion of -Defensins and regenerating islet-derived III (REGIII) proteins. These proteins reduce intestinal pathogens, induce T-reg differentiation, and modulate immune responses by reducing IL-1 and IL-6 expression and increasing IL-10. Nutrition and psychological stress are two of the most significant elements that can directly and indirectly change the microbiota compositions along with other environmental influencing factors. An important regulator of intestinal physiology in the gut-brain-microbiota axis is butyrate, a well-known SCFA. Intestinal dysbiosis, altered intestinal barrier function, behavioral abnormalities, and activation of the hypothalamic–pituitary–adrenal (HPA) axis are all brought on by exposure. Probiotics, bacterial metabolite supplementation, fecal matter transplantation, defined microbial communities, and dietary intervention are some methods for modifying the composition of the gut microbiota that may be used to affect disease-related immune dysfunction and serve as the foundation for a new class of therapeutics.


Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Code Availability
Not applicable.
References
Bouman A, Heineman MJ, Faas MM (2005) Sex hormones and the immune response in humans. Hum Reprod Update 11(4):411–423. https://doi.org/10.1093/humupd/dmi008
Silva MdCNd, Cavalcanti DBA (2019) Evaluation of quality of life in multiple sclerosis patients: impact of fatigue, anxiety and depression. Fisioterapia e Pesquisa 26:339–345. https://doi.org/10.1590/1809-2950/17005426042019
Tullman MJ (2013) Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care 19(2):S15-20
West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P et al (2015) The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol 135(1):3–13. https://doi.org/10.1016/j.jaci.2014.11.012
Brownlee WJ, Hardy TA, Fazekas F, Miller DH (2017) Diagnosis of multiple sclerosis: progress and challenges. The Lancet 389(10076):1336–1346. https://doi.org/10.1016/S0140-6736(16)30959-X
Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15(9):545–558. https://doi.org/10.1038/nri3871
Dobson R, Giovannoni G (2019) Multiple sclerosis–a review. Eur J Neurol 26(1):27–40. https://doi.org/10.1111/ene.13819
Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175(7):4320–4330. https://doi.org/10.4049/jimmunol.175.7.4320
Bahman Y, Maryam M, Aisa B, Falalyeyeva T, Kobyliak N, Majid E (2021) Immunomodulatory role of Faecalibacterium prausnitzii in obesity and metabolic disorders. Minerva Biotechnol Biomol Res. 33(2):76–85
Hemmer B, Kerschensteiner M, Korn T (2015) Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 14(4):406–419. https://doi.org/10.1016/S1474-4422(14)70305-9
Kurschus F (2015) T cell mediated pathogenesis in EAE: Molecular mechanisms. Biomed J 38(3):183–193. https://doi.org/10.4103/2319-4170.155590
Wang C, Gold BG, Kaler LJ, Yu X, Burrows AME, GG, et al (2006) Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem 98(6):1817–1827
Fletcher JM, Lalor S, Sweeney C, Tubridy N, Mills K (2010) T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 162(1):1–11. https://doi.org/10.1111/j.1365-2249.2010.04143.x
Rostami A, Ciric B (2013) Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J Neurol Sci 333(1–2):76–87. https://doi.org/10.1016/j.jns.2013.03.002
Izadi M, Tahmasebi S, Pustokhina I, Yumashev AV, Lakzaei T, Alvanegh AG et al (2020) Changes in Th17 cells frequency and function after ozone therapy used to treat multiple sclerosis patients. Mult Scler Relat Disord 46:102466. https://doi.org/10.1016/j.msard.2020.102466
Glatigny S, Bettelli E (2018) Experimental autoimmune encephalomyelitis (EAE) as animal models of multiple sclerosis (MS). Cold Spring Harb Perspect Med 8(11):a028977. https://doi.org/10.1101/cshperspect.a028977
Markoullis K, Sargiannidou I, Gardner C, Hadjisavvas A, Reynolds R, Kleopa KA (2012) Disruption of oligodendrocyte gap junctions in experimental autoimmune encephalomyelitis. Glia 60(7):1053–1066. https://doi.org/10.1002/glia.22334
Zhu B, Trikudanathan S, Zozulya AL, Sandoval-Garcia C, Kennedy JK, Atochina O et al (2012) Immune modulation by lacto-N-fucopentaose III in experimental autoimmune encephalomyelitis. Clin Immunol 142(3):351–361. https://doi.org/10.1016/j.clim.2011.12.006
Steinman L (2001) Multiple sclerosis: a two-stage disease. Nat Immunol 2(9):762–764. https://doi.org/10.1038/ni0901-762
Haase S, Haghikia A, Wilck N, Müller DN, Linker RA (2018) Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 154(2):230–238. https://doi.org/10.1111/imm.12933
Romijn JA, Corssmit EP, Havekes LM, Pijl H (2008) Gut–brain axis. Curr Opin Clin Nutr Metab Care 11(4):518–521. https://doi.org/10.1097/MCO.0b013e328302c9b0
Powell N, Walker MM, Talley NJ (2017) The mucosal immune system: Master regulator of bidirectional gut–brain communications. Nat Rev Gastroenterol Hepatol 14(3):143–159. https://doi.org/10.1038/nrgastro.2016.191
Seelbach M, Chen L, Powell A, Choi YJ, Zhang B, Hennig B (2010) Polychlorinated biphenyls disrupt blood–brain barrier integrity and promote brain metastasis formation. Environ Health Perspect 118(4):479–484. https://doi.org/10.1289/ehp.0901334
Dinan TG, Cryan JF (2012) Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 37(9):1369–1378. https://doi.org/10.1016/j.psyneuen.2012.03.007
Peerayeh SN, Rostami E, Eslami M, Rezaee MA (2016) High frequency of extended-spectrum β-lactamase-producing klebsiella pneumoniae and escherichia coli isolates from male patients’ urine. Arch Clin Infect Dis 11(2):e60127. https://doi.org/10.5812/archcid.32696
Chapman TM, Plosker GL, Figgitt DP (2006) VSL# 3 probiotic mixture. Drugs 66(10):1371–1387. https://doi.org/10.2165/00003495-200666100-00006
d’Ettorre G, Ceccarelli G, Giustini N, Serafino S, Calantone N, De Girolamo G (2015) Probiotics reduce inflammation in antiretroviral treated, HIV-infected individuals: results of the “probio-HIV” clinical trial. PLoS ONE 10(9):e0137200. https://doi.org/10.1371/journal.pone.0137200
Eslami M, Yousefi B, Kokhaei P, Moghadas AJ, Moghadam BS, Arabkari V (2019) Are probiotics useful for therapy of helicobacter pylori diseases? Comp Immunol Microbiol Infect Dis 64:99–108. https://doi.org/10.1016/j.cimid.2019.02.010
Hemmati M, Yousefi B, Bahar A, Eslami M (2021) Importance of heme oxygenase-1 in gastrointestinal cancers: functions, inductions, regulations, and signaling. J Gastrointest Cancer 52(2):454–461. https://doi.org/10.1007/s12029-021-00587-0
Ghasemian A, Eslami M, Shafiei M, Najafipour S, Rajabi A (2018) Probiotics and their increasing importance in human health and infection control. Rev Med Microbiol 29(4):153–158. https://doi.org/10.1097/MRM.0000000000000147
Eslami M, Bahar A, Hemati M, Rasouli Nejad Z, Mehranfar F, Karami S (2021) Dietary pattern, colonic microbiota and immunometabolism interaction: New frontiers for diabetes mellitus and related disorders. Diabet Med 38(2):e14415. https://doi.org/10.1111/dme.14415
Zhang F, Luo W, Shi Y, Fan Z, Ji G (2012) Should we standardize the 1700-year-old fecal microbiota transplantation? Am J Gastroenterol 107(11):1755. https://doi.org/10.1038/ajg.2012.251
Riccio P, Rossano R (2018) Diet, gut microbiota, and vitamins D+ A in multiple sclerosis. Neurotherapeutics 15(1):75–91. https://doi.org/10.1007/s13311-017-0581-4
Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M (2013) Therapeutic potential of fecal microbiota transplantation. Gastroenterology 145(5):946–953. https://doi.org/10.1053/j.gastro.2013.08.058
Verdier C, Denis S, Gasc C, Boucinha L, Uriot O, Delmas D (2021) An oral FMT capsule as efficient as an enema for microbiota reconstruction following disruption by antibiotics, as assessed in an in vitro human gut model. Microorganisms 9(2):358. https://doi.org/10.3390/microorganisms9020358
Engen PA, Zaferiou A, Rasmussen H, Naqib A, Green SJ, Fogg LF (2020) Single-arm, non-randomized, time series, single-subject study of fecal microbiota transplantation in multiple sclerosis. Front Neurol 8(11):978. https://doi.org/10.3389/fneur.2020.00978
Mohajeri MH, Brummer RJ, Rastall RA, Weersma RK, Harmsen HJ, Faas M (2018) The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr 57(1):1–14. https://doi.org/10.1007/s00394-018-1703-4
Eslami M, Shafiei M, Ghasemian A, Valizadeh S, Al-Marzoqi AH, Shokouhi Mostafavi SK (2019) Mycobacterium avium paratuberculosis and Mycobacterium avium complex and related subspecies as causative agents of zoonotic and occupational diseases. J Cell Physiol 234(8):12415–12421. https://doi.org/10.1002/jcp.28076
Yousefi B, Eslami M, Ghasemian A, Kokhaei P, Sadeghnejhad A (2019) Probiotics can really cure an autoimmune disease? Gene Rep 15:100364. https://doi.org/10.1016/j.genrep.2019.100364
Eslami M, Sadrifar S, Karbalaei M, Keikha M, Kobyliak NM, Yousefi B (2020) Importance of the microbiota inhibitory mechanism on the Warburg effect in colorectal cancer cells. J Gastrointest Cancer 51(3):738–747. https://doi.org/10.1007/s12029-019-00329-3
Wang H, Shi P, Zuo L, Dong J, Zhao J, Liu Q (2016) Dietary non-digestible polysaccharides ameliorate intestinal epithelial barrier dysfunction in IL-10 knockout mice. J Crohns Colitis 10(9):1076–1086. https://doi.org/10.1093/ecco-jcc/jjw065
Salek Farrokhi A, Mohammadlou M, Abdollahi M, Eslami M, Yousefi B (2020) Histone deacetylase modifications by probiotics in colorectal cancer. J Gastrointest Cancer 51(3):754–764. https://doi.org/10.1007/s12029-019-00338-2
Ichimura A, Hirasawa A, Hara T, Tsujimoto G (2009) Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat 89(3–4):82–88. https://doi.org/10.1016/j.prostaglandins.2009.05.003
Tao R, De Zoeten EF, Özkaynak E, Chen C, Wang L, Porrett PM (2007) Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 13(11):1299–1307. https://doi.org/10.1038/nm1652
Wang B, Morinobu A, Horiuchi M, Liu J, Kumagai S (2008) Butyrate inhibits functional differentiation of human monocyte-derived dendritic cells. Cell Immunol 253(1–2):54–58. https://doi.org/10.1016/j.cellimm.2008.04.016
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-y M (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341(6145):569–573. https://doi.org/10.1126/science.1241165
Luu M, Visekruna A (2019) Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol 49(6):842–848. https://doi.org/10.1002/eji.201848009
Schepici G, Silvestro S, Bramanti P, Mazzon E (2019) The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant 28(12):1507–1527. https://doi.org/10.1177/0963689719873890
Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T (2015) Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS ONE 10(9):e0137429. https://doi.org/10.1371/journal.pone.0137429
Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG (2008) The probiotic bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43(2):164–174. https://doi.org/10.1016/j.jpsychires.2008.03.009
Morais LH, Schreiber HL, Mazmanian SK (2021) The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol 19(4):241–255. https://doi.org/10.1038/s41579-020-00460-0
Maltz RM, Keirsey J, Kim SC, Mackos AR, Gharaibeh RZ, Moore CC (2018) Prolonged restraint stressor exposure in outbred CD-1 mice impacts microbiota, colonic inflammation, and short chain fatty acids. PLoS ONE 13(5):e0196961. https://doi.org/10.1371/journal.pone.0196961
Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 7(4):e35240. https://doi.org/10.1371/journal.pone.0035240
Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K (2012) Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol - Gastrointest 303(11):G1288–G1295. https://doi.org/10.1152/ajpgi.00341.2012
Barrett E, Ross R, O’Toole PW, Fitzgerald GF, Stanton C (2012) γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113(2):411–417. https://doi.org/10.1111/j.1365-2672.2012.05344.x
Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK (2017) A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551(7682):648–652. https://doi.org/10.1038/nature24661
Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N (2016) Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res 14(2):127. https://doi.org/10.5217/ir.2016.14.2.127
Amirsaadat S, Jafari-Gharabaghlou D, Alijani S, Mousazadeh H, Dadashpour M, Zarghami N (2021) Metformin and Silibinin co-loaded PLGA-PEG nanoparticles for effective combination therapy against human breast cancer cells. Drug Deliv Sci Technol 61:102107. https://doi.org/10.1016/j.jddst.2020.102107
Samadzadeh S, Mousazadeh H, Ghareghomi S, Dadashpour M, Babazadeh M, Zarghami N (2021) In vitro anticancer efficacy of Metformin-loaded PLGA nanofibers towards the post-surgical therapy of lung cancer. Drug Deliv Sci Technol 61:102318. https://doi.org/10.1016/j.jddst.2020.102318
Maniscalco J, Rinaman L (2018) Vagal interoceptive modulation of motivated behavior. Physiology 33(2):151–167. https://doi.org/10.1152/physiol.00036.2017
Klarer M, Krieger J-P, Richetto J, Weber-Stadlbauer U, Günther L, Winter C (2018) Abdominal vagal afferents modulate the brain transcriptome and behaviors relevant to schizophrenia. J Neurosci Res 38(7):1634–1647. https://doi.org/10.1523/JNEUROSCI.0813-17.2017
Dello Russo C, Lisi L, Feinstein DL, Navarra P (2013) mTOR kinase, a key player in the regulation of glial functions: relevance for the therapy of multiple sclerosis. Glia 61(3):301–311. https://doi.org/10.1002/glia.22433
Keating R, McGargill MA (2016) mTOR regulation of lymphoid cells in immunity to pathogens. Front immunol 7:180. https://doi.org/10.3389/fimmu.2016.00180
Yan J, Wang R, Horng T (2019) mTOR is key to T cell transdifferentiation. Cell Metab 29(2):241–242. https://doi.org/10.1016/j.cmet.2019.01.008
Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S (2018) GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol 11(3):752–762. https://doi.org/10.1038/mi.2017.118
Trifunovic S, Stevanovic I, Milosevic A, Ristic N, Janjic M, Bjelobaba I (2021) The Function of the hypothalamic–pituitary–adrenal axis during experimental autoimmune encephalomyelitis: involvement of oxidative stress mediators. Front Neurosci 17(15):649485. https://doi.org/10.3389/fnins.2021.649485
de Weerth C (2017) Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci Biobehav Rev 83:458–471. https://doi.org/10.1016/j.neubiorev.2017.09.016
Nobuyuki S, Yoichi C, Yuji A, Junko S, Naomi O, Xiao-Nian Y (2004) Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558(1):263–275. https://doi.org/10.1113/jphysiol.2004.063388
Melief J, de Wit SJ, van Eden CG, Teunissen C, Hamann J, Uitdehaag BM (2013) HPA axis activity in multiple sclerosis correlates with disease severity, lesion type and gene expression in normal-appearing white matter. Acta Neuropathol 126(2):237–249. https://doi.org/10.1007/s00401-013-1140-7
Eslami M, Yousefi B (2022) Akkermansia muciniphila as novel powerful bacterial player in the colorectal cancer biotherapeutics. Rev Res Med Microbiol 10:1097. https://doi.org/10.1097/MRM.0000000000000328
Kobyliak N, Falalyeyeva T, Kyriachenko Y, Tseyslyer Y, Kovalchuk O, Hadiliia O (2022) Akkermansia muciniphila as a novel powerful bacterial player in the treatment of metabolic disorders. Minerva Endocrinol 47(2):242–252
Guan Y, Jakimovski D, Ramanathan M, Weinstock-Guttman B, Zivadinov R (2019) The role of Epstein-Barr virus in multiple sclerosis: from molecular pathophysiology to in vivo imaging. Neural Regen Res 14(3):373. https://doi.org/10.4103/1673-5374.245462
Olsson T, Barcellos LF, Alfredsson L (2017) Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol 13(1):25–36. https://doi.org/10.1038/nrneurol.2016.187
Ascherio A, Munger K (2008) Epidemiology of multiple sclerosis: from risk factors to prevention. Semin Neurol 36(2):103–114. https://doi.org/10.1055/s-0036-1579693
Webb A, Kline L, Holick MF (1988) Influence of season and latitude on the cutaneous synthesis of vitamin Dz: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endo-crinol Metab 67(373):378. https://doi.org/10.1210/jcem-67-2-373
Alberto A, Munger KL, Lünemann JD (2012) The initiation and prevention of multiple sclerosis. Nat Rev Neurology 8(11):602–612. https://doi.org/10.1038/nrneurol.2012.198
Ascherio A, Munger KL (2007) Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol 61(4):288–299. https://doi.org/10.1002/ana.21117
Pierrot-Deseilligny C, Souberbielle J-C (2017) Vitamin D and multiple sclerosis: an update. Mult Scler Relat Disord 14:35–45. https://doi.org/10.1016/j.msard.2017.03.014
Sava F, Treszl A, Hajdú J, Toldi G, Rigó J Jr, Tulassay T (2016) Plasma vitamin D levels at birth and immune status of preterm infants. Immunobiology 221(11):1289–1292. https://doi.org/10.1016/j.imbio.2016.06.001
Waubant E, Lucas R, Mowry E, Graves J, Olsson T, Alfredsson L (2019) Environmental and genetic risk factors for MS: an integrated review. Ann Clin Transl Neurol 6(9):1905–1922. https://doi.org/10.1002/acn3.50862
Huppke B, Ellenberger D, Hummel H, Stark W, Röbl M, Gärtner J (2019) Association of obesity with multiple sclerosis risk and response to first-line disease modifying drugs in children. JAMA Neurol 76(10):1157–1165. https://doi.org/10.1001/jamaneurol.2019.1997
Dardiotis E, Tsouris Z, Aslanidou P, Aloizou A-M, Sokratous M, Provatas A (2019) Body mass index in patients with multiple sclerosis: a meta-analysis. Neurol Res 41(9):836–846. https://doi.org/10.1080/01616412.2019.1622873
Mokhtarzade M, Agha-Alinejad H, Motl RW, Negaresh R, Baker JS, Zimmer P (2019) Weight control and physical exercise in people with multiple sclerosis: current knowledge and future perspectives. Complement Ther Med 43:240–246. https://doi.org/10.1016/j.ctim.2019.02.006
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517. https://doi.org/10.1016/S0140-6736(08)61620-7
Karbalaei M, Keikha M, Yousefi B, Ali-Hassanzadeh M, Eslami M (2021) Contribution of aging oral microbiota in getting neurodegenerative diseases. Rev Med Microbiol 32(2):90–94. https://doi.org/10.1097/MRM.0000000000000245
Dargahi N, Katsara M, Tselios T, Androutsou M-E, De Courten M, Matsoukas J (2017) Multiple sclerosis: immunopathology and treatment update. Brain Sci 7(7):78. https://doi.org/10.3390/brainsci7070078
Dong Y, Yong VW (2019) When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat Rev Neurol 15(12):704–717. https://doi.org/10.1038/s41582-019-0253-6
Milovanovic J, Arsenijevic A, Stojanovic B, Kanjevac T, Arsenijevic D, Radosavljevic G (2020) Interleukin-17 in chronic inflammatory neurological diseases. Front Immunol 11:947. https://doi.org/10.3389/fimmu.2020.00947
Matveeva O, Bogie JF, Hendriks JJ, Linker RA, Haghikia A, Kleinewietfeld M (2018) Western lifestyle and immunopathology of multiple sclerosis. Ann N Y Acad Sci 1417(1):71–86. https://doi.org/10.1111/nyas.13583
Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E (2009) Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 132(12):3329–3341. https://doi.org/10.1093/brain/awp289
Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J (2021) A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature 597(7878):709–714. https://doi.org/10.1038/s41586-021-03892-7
Adlravan E, Nejati K, Karimi MA, Mousazadeh H, Abbasi A, Dadashpour M (2021) Potential activity of free and PLGA/PEG nanoencapsulated nasturtium officinale extract in inducing cytotoxicity and apoptosis in human lung carcinoma A549 cells. J Drug Deliv Sci Technol 61:102256. https://doi.org/10.1016/j.jddst.2020.102256
Dibley L, Coggrave M, McClurg D, Woodward S, Norton C (2017) “It’s just horrible”: a qualitative study of patients’ and carers’ experiences of bowel dysfunction in multiple sclerosis. J Neurol 264(7):1354–1361. https://doi.org/10.1007/s00415-017-8527-7
Yousefi B, Kokhaei P, Mehranfar F, Bahar A, Abdolshahi A, Emadi A (2021) The role of the host microbiome in autism and neurodegenerative disorders and effect of epigenetic procedures in the brain functions. Neurosci Biobehav Rev 132:998–1009. https://doi.org/10.1016/j.neubiorev.2021.10.046
Sender R, Fuchs S, Milo R (2016) Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164(3):337–340. https://doi.org/10.1016/j.cell.2016.01.013
Kobyliak N, Falalyeyeva T, Tsyryuk O, Eslami M, Kyriienko D, Beregova T (2020) New insights on strain-specific impacts of probiotics on insulin resistance: evidence from animal study. J Diabetes Metab Disord 19(1):289–296. https://doi.org/10.1007/s40200-020-00506-3
Karbalaei M, Keikha M, Kobyliak NM, Zadeh ZK, Yousefi B, Eslami M (2021) Alleviation of halitosis by use of probiotics and their protective mechanisms in the oral cavity. New Microbes New Infect 42:100887. https://doi.org/10.1016/j.nmni.2021.100887
Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V (2019) Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol 234(10):17127–17143. https://doi.org/10.1002/jcp.28473
Emadi A, Eslami M, Yousefi B, Abdolshahi A (2021) In vitro strain specific reducing of aflatoxin B1 by probiotic bacteria: a systematic review and meta-analysis. Toxin Rev 41(3):995–1006. https://doi.org/10.1080/15569543.2021.1929323
Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV (2005) Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol 237(2):123–130. https://doi.org/10.1016/j.cellimm.2005.11.002
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN (2015) Role of the normal gut microbiota. World J Gastroenterol 21(29):8787. https://doi.org/10.3748/wjg.v21.i29.8787
Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N (2017) Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep 20(6):1269–1277. https://doi.org/10.1016/j.celrep.2017.07.031
Acknowledgements
Department of Bacteriology and Virology, Semnan University of Medical Sciences, Semnan, Iran.
Funding
This research is not supported by a specific project grant.
Author information
Authors and Affiliations
Contributions
ME: Investigate and supervised the findings of this work, wrote the article. Provided critical feedback and helped the analysis of the manuscript, BY: Designed the study, helped supervise the project, and conceived the original idea. Discussed the results and commented on the manuscript. AB: Developed the theoretical framework. Processed the experimental data. SZB and ZKF: Contributed to the final version of the manuscript. Supervised the project, contributed to the interpretation of the results, MD, HG and DP: Contributed to the final version of the manuscript. Supervised the project.
Corresponding author
Ethics declarations
Conflicts of interest
None to declare.
Ethical Approval
The manuscript is a review article and none to declare ethics approval.
Consent to Participate
All individuals taking part in the study gave their informed consent.
Consent for Publication
All individual participants whose identifiable information is used in this article gave their informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yousefi, B., Babaeizad, A., Banihashemian, S.Z. et al. Gastrointestinal Tract, Microbiota and Multiple Sclerosis (MS) and the Link Between Gut Microbiota and CNS. Curr Microbiol 80, 38 (2023). https://doi.org/10.1007/s00284-022-03150-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03150-7