Abstract
Shipment of COVID-19 specimens within the country or overseas at long distances requires cold chain facility using dry ice and triple packing to prevent the risk of COVID-19 infection to the personnel involved in sample transport. The present study aimed to utilize FTA card technology as an alternate means of sample transport and storage across the country. Twenty-one SARS-CoV-2 lab confirmed samples with different Ct value (High, medium & low) were used to detect viral load in samples loaded on FTA card and further compared with VTM samples. The SARS-CoV-2 RNA was detected by rRT-PCR after storing for 14 days at 4 °C and 37 °C. The present study evaluated the utility of FTA cards for preserving the SARS CoV-2 RNA for 14-day period. A significant difference (P < 0.05) was observed in the cycle threshold (ΔCt 4–5) values obtained from FTA and VTM viral samples but it did not affect the positivity. The SARS-CoV-2 RNA could be recovered efficiently from FTA sample stored at 4 °C and 37 °C for 14 days. Thus, FTA cards could be an alternate option for transporting the samples at ambient temperature for a long time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gold standard for the diagnosis of SARS-CoV-2 during the pandemic was the testing of the respiratory samples using real-time PCR. Over a period of time, the other cartridge based real-time platforms like Cepheid and Tru NAT which were originally used for tuberculosis were also used for SARS-CoV-2. The best sample for diagnosis is the nasopharyngeal swab which is collected and transported to the testing laboratories in viral transport media (VTM) in cold chain using frozen ice packs, however, the transport over long distances needs dry ice facility [1]. In case of delay in testing, the samples can be either stored in a refrigerator (short term storage) or −80 °C deep freezers (long term storage) [2,3].
The shipment of hazardous and infectious samples to testing laboratories poses a high risk of infection in terms of spillage during transport and necessitates the use of Personal Protective Equipment (PPE) even by the bearer of the sample and also there is a need to maintain the cold chain [4]. Due to the vast increase in the number of samples being tested, storing even the positive samples in a molecular diagnostic lab remains a great challenge. In such a scenario, sampling on filter papers could be a safe method to transport and store such infectious pathogens.
The Flinders Technology Associates (FTA) classic cards (Whatman, GE healthcare) have been used for a long time to preserve DNA (human & wildlife) and for carrying out the molecular detection and characterization of viral pathogens [5,6,7,8]. Apart from viral diagnosis, sample-loaded FTA cards can also be used for the detection of mutations and genotypes of the pathogens [9]. These cards are known to preserve the nucleic acid and are impregnated with chaotropic agents which fix, inactivate the viruses and at the same time stabilizes the nucleic acid [10,11]. The testing using these cards can be carried out in small sample volumes and these can be stored at room temperature for a long time. The RNA stability on FTA cards has been observed for 5 months at room temperature for avian influenza virus [12]. This stability can further be increased by the storage of these cards in − 80 °C deep freezers [13].
The use of such an alternative method for sample collection, storage, and transport would eliminate the need for a cold chain and thereby can enhance the diagnosis and surveillance of SARS-CoV-2 in resource-limited laboratories during the COVID-19 pandemic [14]. The latest study on SARS-CoV-2 used four punches of 6 mm disk of FTA cards which were loaded with swab dipped in saline. However, the study did not mention the viral load or Ct value of the sample used [15]. The present study aimed to assess the RNA recovery and stability of SARS-CoV-2 samples on FTA cards stored at 4 °C and 37 °C. These temperature conditions were chosen to simulate the natural environmental conditions in tropical countries like India where it varies from 18 to 48 °C. Also, the amplification and sequencing of a large amplicon (approximately 777 bp region including full ORF 8 gene) was performed to establish an alternative approach for the detection and molecular characterization of SARS-CoV-2 virus.
Materials and Methods
-
1.
Site: The study was performed in the Department of Virology, Postgraduate Institute of Medical Education and Research (PGIMER), a referral tertiary care hospital in North India.
-
2.
Sample Collection and Processing: The nasopharyngeal swabs collected in viral transport medium from SARS-CoV-2 infected patients received as a part of routine diagnosis in the department were used for the study.
-
3.
Ethical Approval: The study was duly approved by the Post Graduate Institute of Medical Education and Research (PGIMER) Ethical Committee as per National guidelines vide letter No. NK/6446/Study/684 dated 14.07.2020.
-
4.
Sample Selection Criteria: Samples already tested positive for SARS-CoV-2 were selected and categorized based on the Ct value of envelope (E) and spike (S) genes in real-time PCR. Samples with Ct value < 21, 22–27, and > 27 was considered as low, medium, and high Ct value samples, respectively [16].
FTA® Cards
Whatman® FTA® Classic cards (WHAWB120205) were used for study per se and stored at room temperature as per manufacturer’s instructions [17]. A single card contained four circles and 125 µl of the samples were loaded on each circle of the FTA card and left to dry at room temperature for 1 h. The cards were punched with an office puncher (4 mm) in class II A2 biosafety cabinet and processed for RNA extraction. All precautions were taken to prevent carry-over contamination and in between the various samples by cleaning the punch machine with 70% alcohol.
Experimental Design
Three types of experiments were designed to optimize and standardized the SARS-CoV-2 RNA on FTA cards.
-
a)
Optimization of Disk Punched: Three SARS-CoV-2 positive samples from each category i.e., low, medium and high Ct value samples were used for this experiment. A total of 125 μl of each sample was loaded on each FTA card circle and left to dry at room temperature for 1 h and thereafter stored at 4 °C for one day. Post one day the cards were punched with 4 mm office puncher. To optimize the number of disks required for RNA detection, 4, 8 and 12 disk (approximately half circle) were punched from the FTA cards. And disks were subjected to rRT-PCR and Conventional PCR following RNA extraction from cards. (Fig. 1a).
-
b)
RNA Recovery from FTA Cards: To determine the SARS-CoV-2 RNA recovery from FTA cards, a high positive sample (Ct value- E- 11.74 & S- 12.43) of known concentration (3.7 × 104copies/µL) was ten-fold serially diluted and 125 µl of each dilution was loaded on one circle. Post one day storage at 4 °C, 12 disks of 4 mm diameter were punched from cards of each dilution and processed for RNA elution and SARS-CoV-2 RNA detection by both conventional RT-PCR and rRT-PCR to determine the amount of RNA recovery from FTA cards. The RNA detection of VTM sample dilutions were also performed post day 1 storage at 4 °C (Fig. 1b).
-
c)
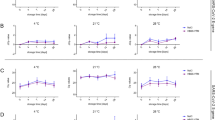
Stability of RNA on FTA Cards: To detect the stability of SARS-CoV-2 RNA on FTA cards, seven samples from each category (low, medium, and high Ct value) were loaded on 126 cards circles (7samples × 3categories × 2 temperature × 3 time points). Each cards were packed in biohazard plastic pouches and kept at 4 °C and 37 °C. The SARS-CoV-2 RNA detection was done on day 1, 7 and 14 (Fig. 1c).
RNA Elution
The RNA was extracted using the commercially available kit (Qiagen, Germany, cat. 52,906) with slight modifications in the protocol provided by the manufacturer. The FTA punches were transferred to the tube and 200 µl of phosphate buffer saline was added followed by the addition of 560 µl of AVL buffer with carrier RNA. The tube was vortexed followed by incubation at room temperature of 10 min, quick spin, and transfer of supernatant to a clean tube, thereafter manufacturer instructions were followed. Also, for comparison purpose, RNA was directly extracted from 125 µl of the VTM samples using the same kit.
Real-Time PCR
The SARS-CoV-2 RNA detection was done by real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) using Real Star® SARS-CoV-2 RT-PCR Kit 1.0 (Altona Diagnostics, Germany, cat. 821,005) which detects E (screening) and S gene (confirmatory) of SARS-CoV-2. To determine the viral load, a positive control with known concentration of 104 copies/µl received from Altona Diagnostics Pvt Ltd, Germany was diluted serially for the preparation of standard curve.
RT-PCR and Sequencing
Conventional RT-PCR was performed by targeting the ORF-8 gene (confirmatory gene) using primers designed by Primer BLAST software. The forward primer with a sequence of 5’TTTTAGCCTTTCTGCTATTCCTTG 3’ and reverse primer with a sequence of 5’CCGTCACCACCACGAATTC 3’ was used for amplification with reverse transcription step at 50 °C for 30 min, initial denaturation at 95 °C for 2 min and 40 cycles of denaturation (95 °C, 15 s), annealing (62 °C, 30 s) and extension (72 °C, 1 min). The amplified product of 777 bp was purified using High Yield Gel/PCR DNA extraction kit (Real Biotech Corporation, Taiwan, Cat. QDF100) as per the manufacturer’s protocol and was used for Sanger sequencing.
Statistical Analysis
Correlation and inferential tests were performed using SPSS Statistics software version 20 (IBM Corporation, Armonk, NY, USA). Student t-test was used to compare the difference in Ct values obtained from VTM and FTA viral samples stored at different temperatures. The Ct values obtained for FTA samples were also compared with the original sample at different temperatures stored for 14 days by one-way ANOVA followed by postdoc Bonferroni analysis. Pearson’s correlation was performed to predict the stability of SARS-CoV-2 RNA on FTA stored at different temperatures (4 °C and 37 °C) for 14 days. A p value < 0.05 was considered statistically significant.
Results
Optimization of Disk Punched
During optimization of disks punched for the three categories of low, medium and high Ct value samples, it was observed that the results were similar for 8 and 12 disks compared to 4 disks. (Table 1; Fig S1). However, compared to Ct value of positive sample reduction was observed in all the three disks. While performing conventional PCR of ORF-8 from the FTA cards, only the low Ct value (< 21) sample was detected in all the three disks protocol and 12 disk protocol showed a sharper band at 777 bp as compared to the 4 and 8 disk (Fig. 2). Hence all further experiments were conducted with 12 disks protocol.
RNA Recovery From FTA Cards
The serial dilutions of high positive sample were subjected to SARS-CoV-2 RNA detection in both VTM and FTA cards when compared post one day storage at 4 °C. The RNA up to 6 times log dilution (10–6) was detected on FTA cards however, results were not reproducible in 7 times log dilution (10–7). Also, 4–6 Ct value difference was observed in both genes up to 10–4 sample dilutions in VTM and FTA. The Ct value of E and S gene in VTM and FTA dilutions are shown in Table 2. The log dilution of VTM and FTA samples were also tested by conventional PCR targeting ORF-8 gene. The VTM samples at day 1 were detected up to 6 times log dilution (10–6; equivalent to 29.60 Ct value) whereas in case of FTA day 1, only up to 2 times log dilution (10–2; equivalent to 21.90 Ct value) as depicted in Fig. 3 & Table 2.
SARS-CoV-2 RNA recovery from FTA and VTM sample dilutions after ten-fold serial dilution of known positive sample (3.7 × 104 copies/µl). Lanes 1–8: dilution corresponding to neat, 10–1, 10–2, 10–3, 10–4, 10–5, 10–6, 10–7 of stock. MM represents Molecular Marker (100 bp) & amplified ORF-8 band corresponds to 777bp.
Stability of RNA on FTA Cards
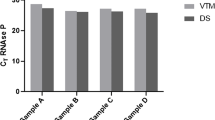
On stability testing at 4 °C & 37 °C on day 1, 7 and 14; SARS-CoV-2 RNA for both genes were detected in all the categories of samples. Compared with the original VTM sample, Ct values of FTA samples on day 1 were significantly increased while no significant increase in Ct value was observed on day 7 and day 14. Further no difference in Ct values was observed at different temperatures (Table 3; S1 Table, Fig S2-S3). Moreover, correlation studies were also performed between the 4 °C and 37 °C at three-time points for both the genes and a positive correlation was observed between the two-temperature (Fig. 4) indicating the good stability and utility of FTA cards between temperature 4 °C-37 °C maximum up to day 14 post-loading of samples.
The sensitivity of SARS-CoV-2 on FTA was also calculated at 4 °C and 37 °C on day 14. At 4 °C the percentage positivity was 90.47% (19/21) when either gene (E or S) was detected and 85.71% (18/21) when both the genes (E and S) were detected. Whereas at 37 °C the percentage positivity was the same i.e., 85.71% (18/21) in both cases. (Fig. 5).
The SARS-CoV-2 RNA eluted from FTA samples was used for ORF 8 gene amplification and it was found that the integrity of RNA was maintained and can be used for sequencing purposes and genotyping analysis. The sequencing results were submitted for ORF 8 gene in NCBI (MZ948892) and chromatogram was shown in supplementary information (Fig S4).
Discussion
The bottleneck for the sample transportation from field to the testing lab in case of RNA viruses is cold chain and dry ice which is mandatory and expensive. Considering the resource limiting set up for various labs during pandemic, the availability of cold chain and dry ice is itself an issue. Hence, there is an urgent need for a collection technique which do not require any cold chain and can facilitate sample transportation and maintaining the RNA viability to an extent that the sequencing of RNA can also be performed for the early detection of variants. Moreover, the testing labs receive a large number of samples and due to space constraints, the samples storage even for positive samples is again a huge problem. Hence, there is an urgent need for an alternate strategy for sample storage without affecting the integrity of RNA.
FTA cards is such a technique that was started for the detection of DNA but soon shifted for the detection of many RNA viruses [18,19,20]. FTA cards are easy to access, safe (non-infectious), and shipment at ambient temperature possible. Also, minimal storage space required for FTA samples compared to VTM samples. FTA disk has also been used for the QA/QC of measles rubella wherein the samples are shipped to various labs for genotyping. The present study focuses on the utility of FTA cards as an alternate option for the transportations and storage of highly infectious SARS-CoV-2 samples.
The present study confirmed that the detection of SARS-CoV-2 RNA is possible from FTA cards. Twelve disks of 4 mm punch which is equivalent to 50% card area could detect samples with a high Ct value of more than 27. Disk numbers may vary depending upon the viral load of the sample-loaded on to these disks hence disk standardization for different viruses and type of sample is required. In this study, a greater number of disks were required as the processing was done directly from the NPS samples with varying Ct value rather than cell culture isolates. The latest study on SARS-CoV-2 used four punches of 6 mm disk of FTA cards which were loaded with swab dipped in saline. However, the study did not mention the viral load or Ct value of the sample used [15].
The RNA up to 6 times log dilution (10–6) was detected on FTA cards at both day 1, results were not reproducible in 10–7 dilution. The same results were obtained for VTM dilutions. The results of both samples in VTM as well as FTA cards are comparable, hence it is unlikely that FTA cards will miss low positive samples. However, if FTA cards are to be used for genotyping, the same will be possible only if the viral load of the sample is high as the end point PCR displayed sharp bands up to 2-time log dilution (10–2) in FTA compared to VTM which were detected up to 6 times log dilution (10–6). A recent study revealed feasibility of FTA card for HIV viral load testing and showed good correlation between FTA cards and plasma samples [21]
The present study tested the SARS-CoV-2 RNA stability for 14 days based on the consideration that this duration will be sufficient for the transportation of samples within the country and overseas. The study inferred that SARS-CoV-2 RNA is stable on FTA cards for 14 days post-inoculation which is in agreement with the latest study on SARS-CoV-2 [15]. Some other studies have also reported the detection of viral RNA on FTA for up to 150 days [4,5,22] though long storage on FTA may affect RNA integrity and sensitivity [23,24]. In the present study, the sensitivity was reported to be between 85 and 90% based on both screening and confirmatory genes. The sensitivity of throat swabs and oral fluid of measles and rubella virus on FTA has been reported to be 79.4% and 85.5%, respectively [25]. The sensitivity of RNA on FTA was found to decrease over a period of 14 days (5–7 Ct value change) possibly due to RNA denaturation [23,24].
In this study, SARS-CoV-2 RNA was found to be stable at 4 °C and 37 °C showing a good correlation of Ct values between the two temperatures hence concluding that FTA cards with SARS-CoV-2 sample can be transported at any temperature between 4 and 37 °C which has relevance especially in tropical countries. These two temperatures were selected as 4 °C is optimum for sample transportation of RNA virus and 37 °C was selected as environmental temperature for temperate and tropical countries is around 37 °C. The results were in accordance with previous work on SARS-CoV-2 where the RNA was stable at different temperature (− 20 °C, 4–8 °C, RT) [15]. Moreover, the stability of RNA on FTA is well observed for other viruses also at different temperatures [4,5,6,22,23].
The present experimental study is distinct from other studies on FTA as the authors have standardized and optimized the detection of SARS-CoV-2 RNA with the varying range of Ct value samples including those with low copy number. It was observed that even the high Ct value samples (greater than 27) which could also be detected after inoculation on FTA cards. Also, the authors tried to show the intact integrity of RNA by conventional methods and utility of FTA stored samples for sequencing and other genotyping purposes.
The limitations of our study were (a) small sample size owing to limited resources (b) the clinical samples collected in VTM were inoculated on the cards rather than the direct patient samples being collected on these cards (c) viability of SARS-CoV-2 on FTA cards samples could not be assessed as BSL 3 facility was not available so could not comment on infectiousness of FTA samples. However, in a systematic review by Cardona et al., 2019, it was shown that out of 14 studies which tested the inactivation of FTA, the infectivity was demonstrated in only one case [26]. In our preliminary experiment it was seen that SARS-CoV-2 RNA was not detected when the dry nasopharyngeal swab was rubbed on FTA cards, hence revealing the importance of a liquid medium before the samples is instilled on FTA. Hence, normal saline or phosphate buffer saline may be used as an alternative for VTM which will cut down the expenses in resource limiting setting.
Overall, our study suggest that FTA cards can be used for the detection of SARS-CoV-2 RNA even after 14 days of sample collection at temperature stored at 4 °C and 37 °C with a wide range of Ct value sample.
Data Availability
Available when required.
Code Availability
NA.
References
National centre for disease control. specimen collection, packaging and transport guidelines for 2019 nCoV - Acute Respiratory Disease. https://ncdc.gov.in/WriteReadData/l892s/50471431021580628750.pdf , 2020 (Accessed 30 July 2020).
World Health Organization. instructions for storage and transport of suspected or confirmed human and animal specimens and virus isolates of pandemic (H1N1) 2009. https://www.who.int/influenza/gisrs_laboratory/logistic_activities/transport_storage_specimens_isolates/en/ 2020 (Accessed 13 July 2020).
Johnson FB (1990) Transport of viral specimens. Clin Microbiol Rev 3:120–131
Jóźwiak M, Wyrostek K, Domańska-Blicharz K, Olszewska-Tomczyk M, Śmietanka K, Minta Z (2016) Application of FTA® cards for detection and storage of avian influenza virus. J Vet Res 60:1–6
Muthukrishnan M, Singanallur NB, Ralla K, Villuppanoor SA (2008) Evaluation of FTA® cards as a laboratory and field sampling device for the detection of foot-and-mouth disease virus and serotyping by RT-PCR and real-time RT-PCR. J Virol Methods 151:311–316
Perozo F, Villegas P, Estevez C, Alvarado I, Purvis LB (2006) Use of FTA® filter paper for the molecular detection of Newcastle disease virus. Avian Pathol 35:93–98
Katz RS, Premenko-Lanier M, McChesney MB, Rota PA, Bellini WJ (2002) Detection of measles virus RNA in whole blood stored on filter paper. J Med Virol 67:596–602
Smith L, Burgoyne LA (2004) Collecting, archiving and processing DNA from wildlife samples using FTA® databasing paper. BMC Ecol 4:4
Sierra-Arguello Y, Faulkner O, Tellez G, Hargis B, do Nascimento VP. (2016) The use of FTA cards for transport and detection of gyr A mutation of Campylobacter jejuni from poultry. Poult sci 95:798–801
Rajendram D, Ayenza R, Holder F, Moran B, Long T, Shah H (2006) Long-term storage and safe retrieval of DNA from microorganisms for molecular analysis using FTA matrix cards. J Microbiol Methods 67:582–592
Abe K, Konomi N (1998) Hepatitis C virus RNA in dried serum spotted onto filter paper is stable at room temperature. J Clin Microbiol 36:3070–3072
Abdelwhab E, Lüschow D, Harder TC, Hafez HM (2011) The use of FTA® filter papers for diagnosis of avian influenza virus. J Virol Methods 174:120–122
Sakai T, Ishii A, Segawa T, Takagi Y, Kobayashi Y, Itou T (2015) Establishing conditions for the storage and elution of rabies virus RNA using FTA® cards. J Vet Med Sci 77:461–465
Chauhan P, Gupta P, Chhikara K, Goyal K, Singh MP (2021) FTA cards for COVID 2019 samples: easy and cost effective innovation! VirusDisease 32:20–21
Kriegshäuser G, Enko D, Reçi L, Leb CM, Panhofer P (2021) Stability of SARS-CoV-2 RNA in FTA card spot-prep samples derived from nasopharyngeal swabs. Clin Chem Lab Med (CCLM) 58(9):e351–e353
Chhikara K, Kanta P, Ghosh A, Prakash RC, Goyal K, Singh MP (2020) Validation of SARS CoV-2 detection by real-time PCR in matched pooled and deconvoluted clinical samples before and after nucleic acid extraction: a study in tertiary care hospital of North India. Diagn Microbiol Infect Dis 99:115206
Whatman® FTA™ card technology. https://www.sigmaaldrich.com/IN/en/product/sigma/whawb120205 (Accessed 15 July 2022).
Picard-Meyer E, Barrat J, Cliquet F (2007) Use of filter paper (FTA®) technology for sampling, recovery and molecular characterisation of rabies viruses. J Virol Methods 140:174–182
Purvis LB, Villegas P, Perozo F (2006) Evaluation of FTA® paper and phenol for storage, extraction and molecular characterization of infectious bursal disease virus. J Virol Methods 138:66–69
Beck IA, Drennan KD, Melvin AJ, Mohan KM, Herz AM, Alarcón J, Piscoya J, Velázquez C, Frenkel LM (2001) Simple, sensitive, and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J Clin Microbiol 39:29–33
Yacouba A, Congo M, Dioma GK, Somlare H, Coulidiaty D, Ouattara K, Whatman Sangare L (2020) FTA cards versus plasma specimens for the quantitation of HIV-1 RNA using two real-time PCR assays. Access Microbiol. https://doi.org/10.1099/acmi.0.000138
Keeler SP, Ferro PJ, Brown JD, Fang X, El-Attrache J, Poulson R, Jackwood MW, Stallknecht DE (2012) Use of FTA® sampling cards for molecular detection of avian influenza virus in wild birds. Avian Dis 56:200–207
Moscoso H, Raybon EO, Thayer SG, Hofacre CL (2005) Molecular detection and serotyping of infectious bronchitis virus from FTA® filter paper. Avian Dis 49:24–29
Dobbs LJ, Madigan MN, Carter AB, Earls L (2002) Use of FTA gene guard filter paper for the storage and transportation of tumor cells for molecular testing. Arch Pathol Lab Med 126:56–63
Bankamp B, Sein C, Simbu EP, Anderson R, Abernathy E, Chen M-H, Tamfum J-JM, Wannemuehler KA, Waku-Kouomou D, Lopareva EN (2019) Use of FTA cards to transport throat swabs and oral fluid samples for molecular detection and genotyping of measles and rubella viruses. J clin microbiol 57(5):e00048-19
Cardona-Ospina JA, Villalba-Miranda MF, Palechor-Ocampo LA, Mancilla LI, Sepúlveda-Arias JC (2019) A systematic review of FTA cards® as a tool for viral RNA preservation in fieldwork: Are they safe and effective? Prev Vet Med 172:104772
Acknowledgements
We acknowledge Altona Diagnostics India Pvt Ltd. for providing positive control of known concentration. This work was carried out as a part of routine COVID-19 diagnostic activity of the Regional Virus Diagnostic Laboratory under ICMR New Delhi, by the Department of Virology, PGIMER, Chandigarh.
Author information
Authors and Affiliations
Contributions
Conceptualization- PK; Data curation- PK and KC; Funding acquisition-, MS; Investigation- AG and KG; Methodology, LM; Project administration- MS; Supervision- AG and MS; Writing – original draft- KC; Writing – review & editing- KG.
Corresponding author
Ethics declarations
Conflict of interest
Nothing to declare.
Ethical Approval
The study was approved by host institute with IEC No. NK/6446/Study/684 dated 14.07.2020.
Consent for Publication
NA
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanta, P., Chhikara, K., Mohan, L. et al. Alternate Approach in Storing and Shipment of SARS-CoV-2 RNA Samples with the Use of FTA Cards. Curr Microbiol 79, 396 (2022). https://doi.org/10.1007/s00284-022-03079-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03079-x