Abstract
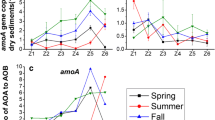
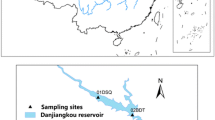
Ammonia-oxidizing bacteria (AOB) play an important role in nitrification in estuaries. The aim of this study was to examine the spatial abundance, diversity, and activity of AOB in coastal sediments of the Liaohe Estuary using quantitative PCR, high-throughput sequencing of the amoA gene coding the ammonia monooxygenase enzyme active subunit, and sediment slurry incubation experiments. AOB abundance ranged from 8.54 × 104 to 5.85 × 106 copies g−1 of wet sediment weight and exhibited an increasing trend from the Liaohe Estuary to the open coastal zone. Potential nitrification rates (PNRs) ranged from 0.1 to 336.8 nmol N g−1 day−1 along the estuary to the coastal zone. Log AOB abundance and PNRs were significantly positively correlated. AOB richness decreased from the estuary to the coastal zone. High-throughput sequencing analysis indicated that the majority of amoA gene sequences fell within the Nitrosomonas and Nitrosomonas-like clade, and only a few sequences were clustered within the Nitrosospira clade. This finding indicates that the Nitrosomonas-related lineage may be more adaptable to the specific conditions in this estuary than the Nitrosospira lineage. Sites with high nitrification rates were located in the southern open region and were dominated by the Nitrosomonas-like lineage, whereas the Nitrosospira lineage was found primarily in the northern estuary mouth sites with low nitrification rates. Thus, nitrification potentials in Liaohe estuarine sediments in the southern open region were greater than those in the northern estuary mouth, and the Nitrosomonas-related lineage might play a more important role than the Nitrosospira lineage in nitrification in this estuary.





Similar content being viewed by others
References
Bai J, Chen CT, Zhao YG, Tian WJ, Dong X, Yin NN (2010) Studies on nitrobacteria and nitrification in Liaohe estuary wetland sediments. Environ Sci 31(12):3011–3017 (Chinese)
Bernhard A (2010) The Nitrogen cycle: processes, players, and human impact. Nat Educ Knowl 3(10):25
Bernhard AE, Bollmann A (2010) Estuarine nitrifiers: New players, patterns and processes. Estuar Coastal Shelf Sci 88(88):1–11
Bernhard AE, Donn T, Giblin AE, Stahl DA (2005) Loss of diversity of ammonia-oxidizing bacteria correlates with increasing salinity in an estuary system. Environ Microbiol 7(9):1289–1297
Bernhard AE, Tucker J, Giblin AE, Stahl DA (2007) Functionally distinct communities of ammonia-oxidizing bacteria along an estuarine salinity gradient. Environ Microbiol 9(6):1439–1447
Cao H, Hong Y, Li M, Gu JD (2012) Community shift of ammonia-oxidizing bacteria along an anthropogenic pollution gradient from the Pearl River Delta to the South China Sea. Appl Microbiol Biotechnol 94(1):247–259
Chen LG, Fan JF, Guan DM, Zhao HD, Ming HX, Wu LJ, Chen JY (2010) Analysis of temporal and spatial distribution of nitrobacteria in sediment of Liaohe Estuary. Mar Environ Sci 29(2):174–178 (Chinese)
Chen Y, Zhen Y, He H, Lu X, Mi T, Yu Z (2014) Diversity, abundance, and spatial distribution of ammonia-oxidizing β-proteobacteria in sediments from Changjiang Estuary and its adjacent area in East China Sea. Microb Ecol 67(4):788–803
Damashek J, Smith JM, Mosier AC, Francis CA (2015) Benthic ammonia oxidizers differ in community structure and biogeochemical potential across a riverine delta. Front Microbiol 5:743
Dong X, Reddy GB (2012) Ammonia-oxidizing bacterial community and nitrification rates in constructed wetlands treating swine wastewater. Ecol Eng 40(3):189–197
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J (2014) An improved dual-indexing approach for multiplexed 16 S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2 (1):1–7. doi:10.1186/2049-2618-2-6
Francis CA, O’Mullan GD, Ward BB (2003) Diversity of ammonia monooxygenase (amoA) genes across environmental gradients in Chesapeake Bay sediments. Geobiology 1(2):129–140
Freitag TE, Chang L, Prosser JI (2006) Changes in the community structure and activity of betaproteobacterial ammonia-oxidizing sediment bacteria along a freshwater-marine gradient. Environ Microbiol 8(4):684–696
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (2008) The specification for marine monitoring-Part 4: seawater analysis. Standards Press of China, Beijing
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (2008) The specification for marine monitoring-Part 5: sediment analysis. Standards Press of China, Beijing
Head IM, Hiorns WD, Embley TM, Mccarthy AJ, Saunders JR (1993) The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16 S ribosomal RNA gene sequences. J Gen Microbiol 139(6):1147–1153
Henriksen K (1980) Measurement of in situ rates of nitrification in sediment. Microb Ecol 6(4):329–337
Horz HP, Barbrook A, Field CB, Bohannan BJ (2004) Ammonia-oxidizing bacteria respond to multifactorial global change. Proc Nat Acad Sci USA 101(42):15136–15141
Jensen K, Sloth NP, Risgaard-Petersen N, Rysgaard S, Revsbech NP (1994) Estimation of nitrification and denitrification from microprofiles of oxygen and nitrate in model sediment systems. Appl Environ Microb 60(6):2094–2100
Ji Y, Zhou G, New T (2009) Abiotic factors influencing the distribution of vegetation in coastal estuary of the Liaohe Delta, Northeast China. Estuar Coasts 32 (5):937–942
Jin T, Zhang T, Ye L, Lee OO, Yue HW, Qian PY (2011) Diversity and quantity of ammonia-oxidizing Archaea and Bacteria in sediment of the Pearl River Estuary, China. Appl Microbiol Biotechnol 90(3):1137–1145
Junier P, Molina V, Dorador C, Hadas O, Kim OS, Junier T, Witzel KP, Imhoff JF (2010) Phylogenetic and functional marker genes to study ammonia-oxidizing microorganisms (AOM) in the environment. Appl Microbiol Biotechnol 85(3):425–440
Li J, Nedwell DB, Beddow J, Dumbrell AJ, Mckew BA, Thorpe EL, Whitby C (2015) amoA Gene abundances and nitrification potential rates suggest that benthic ammonia-oxidizing bacteria and not Archaea dominate N cycling in the Colne Estuary, United Kingdom. Applied &. Environ Microbiol 81(1):159–165
Li Z, Jin W, Liang Z, Yue Y, Lv J (2013) Abundance and diversity of ammonia-oxidizing archaea in response to various habitats in Pearl River Delta of China, a subtropical maritime zone. J Environ Sci 25(6):1195–1205
Liu B, Li Y, Zhang J, Zhou X, Wu C (2014) Abundance and diversity of ammonia-oxidizing microorganisms in the sediments of Jinshan Lake. Curr Microbiol 69(5):751–757
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963
Men B, He MC, Li T, Lin CY, Quan XC (2009) Distributions of polycyclic aromatic hydrocarbons in the Daliao River Estuary of Liaodong Bay, Bohai Sea (China). Mar Pollut Bull 58(6):818–826
Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10(11):3002–3016
Purkhold U, Wagner M, Timmermann G, Pommerening-Röser A, Koops HP (2003) 16 S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. Int J Syst Evol Microbiol 53(5):1485–1494
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Sims A, Gajaraj S, Hu Z (2012) Seasonal population changes of ammonia-oxidizing organisms and their relationship to water quality in a constructed wetland. Ecol Eng 40(3):100–107
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5 : molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol Int J org Evol 28(10):2731–2739
Tsiknia M, Paranychianakis NV, Varouchakis EA, Nikolaidis NP (2015) Environmental drivers of the distribution of nitrogen functional genes at a watershed scale. Fems Microbiol Ecol 91 (6):fiv052
Wankel SD, Mosier AC, Hansel CM, Paytan A, Francis CA (2011) Spatial variability in nitrification rates and ammonia-oxidizing microbial communities in the agriculturally impacted Elkhorn Slough estuary, California. Appl Environ Microbiol 77(1):269–280
Zhang JH, Yu LX, Yao QZ, Tian L (2014) Mixing behavior of nutrients in different seasons at Liaohe Estuary. Environ Sci 35(2):569–576 (Chinese)
Zhang Y, Chen L, Sun R, Dai T, Tian J, Wen D (2015) Ammonia-oxidizing bacteria and archaea in wastewater treatment plant sludge and nearby coastal sediment in an industrial area in China. Appl Microbiol Biotechnol 99(10):4495–4507
Zhao D, Zeng J, Wan W, Liang H, Huang R, Wu QL (2013) Vertical distribution of ammonia-oxidizing archaea and bacteria in sediments of a Eutrophic Lake. Curr Microbiol 67(3):327–332
Zheng Y, Hou L, Min L, Min L, Hui Z, Yin G, Zhou J (2013) Diversity, abundance, and activity of ammonia-oxidizing bacteria and archaea in Chongming eastern intertidal sediments. Appl Microbiol Biotechnol 97(18):8351–8363
Zheng Y, Hou L, Newell S, Liu M, Zhou J, Zhao H, You L, Cheng X (2014) Community dynamics and activity of ammonia-oxidizing prokaryotes in intertidal sediments of the Yangtze estuary. Appl Environ Microbiol 80(1):408–419
Acknowledgements
We thank Hongjun Li and Chunxin Zhang for collecting the sediment samples and physiochemical data during the spring cruise. This work was supported by the National Natural Science Foundation (Grant 41676115), the National Key Research Program (Grant 2016YFA0601400), and the State Oceanic Administration (Grant GASI-03-01-02-05) of China. This study is a contribution to the international IMBER project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, Y., Fan, J., Su, J. et al. Spatial Abundance, Diversity, and Activity of Ammonia-Oxidizing Bacteria in Coastal Sediments of the Liaohe Estuary. Curr Microbiol 74, 632–640 (2017). https://doi.org/10.1007/s00284-017-1226-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1226-x