Abstract
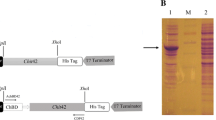
The antagonism of Trichoderma strains usually correlates with the secretion of fungal cell wall degrading enzymes such as chitinases. Chitinase Chit42 is believed to play an important role in the biocontrol activity of Trichoderma strains as a biocontrol agent against phytopathogenic fungi. Chit42 lacks a chitin-binding domain (ChBD) which is involved in its binding activity to insoluble chitin. In this study, a chimeric chitinase with improved enzyme activity was produced by fusing a ChBD from T. atroviride chitinase 18–10 to Chit42. The improved chitinase containing a ChBD displayed a 1.7-fold higher specific activity than chit42. This increase suggests that the ChBD provides a strong binding capacity to insoluble chitin. Moreover, Chit42-ChBD transformants showed higher antifungal activity towards seven phytopathogenic fungal species.



Similar content being viewed by others
References
Alfthan K, Takkinen K, Sizmann D et al (1995) Properties of a single-chain antibody containing different linker peptides. Protein Eng 8:725–731
Arakane Y, Zhu Q, Matsumiya M, Muthukrishnan S et al (2003) Properties of catalytic, linker and chitin-binding domains of insect chitinase. Insect Biochem Mol Biol 33:631–648
Aranaz I, Mengı′bar M, Harris R, Panos I, Miralles B et al (2009) Functional characterization of chitin and chitosan. Curr Chem Biol 3:203–230
BollerT Mauch F (1988) Colorimetric assay for chitinase. Methods Enzymol 161:430–435
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:249–254
Chern JT, Chao YP (2005) Chitin-binding domain based immobilization of d-hydantoinase. J Biotechnol 117:267–275
De la Cruz J, Hidalgo-Gallego A, Lora JM, Benítez T et al (1992) Isolation and characterization of three chitinases from Trichoderma harzianum. Eur J Biochem 206:859–867
Delgado-Jarana J, Rincon AM, Benitez T (2002) Aspartyl protease from Trichoderma harzianum CECT 2413: cloning and characterization. Microbiology 148:1305–1315
Fan Y, Fang W, Guo S, Pei X et al (2007) Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl Environ Microbiol 73:295–302
García I, Lora JM, De la Cruz J, Benítez T et al (1994) Cloning and characterization of a chitinase (CHIT42) cDNA from the mycoparasitic fungus Trichoderma harzianum. Curr Genet 27:83–89
Glass NL, Donaldson G (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Guthrie JL, Khalif S, Castle AJ (2005) An improved method for detection and quantification of chitinase activities. Can J Microbiol 51:491–495
Hardt M, Laine RA (2004) Mutation of active site residues in the chitin-binding domain ChBDChiA1 from chitinase A1 of Bacillus circulans alters substrate specificity: use of a green fluorescent protein binding assay. Arch Biochem Biophys 426:286–297
Hashimoto M, Ikegami T, Seino S, Ohuchi N et al (2000) Expression and characterization of the chitin-binding domain of chitinase A1 from Bacillus circulans WL-12. J Bacteriol 182:3045–3054
Kubicek CP, Herrera-Estrella A, Seidl-Seiboth V, Martinez DA, Druzhinina IS, Thon M, Zeilinger S et al (2011) Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol 12:R40
Lee SB, Taylor JW (1990) Isolation of DNA from fungal mycelia and single spores. In: Innis DGM, Sninsky J, White T (eds) In: PCR protocols: a guide to methods and applications, vol 34. Academic Press, Orlando
Limón MC, Lora JM, García I, De la Cruz J, Llobell A et al (1995) Primary structure and expression pattern of the 33 kDa chitinase gene from the mycoparasitic fungus Trichoderma harzianum. Curr Genet 28:478–483
Limon MC, Margolles-Clark E, Benitez T, Penttila M (2001) Addition of substrate-binding domains increases substrate-binding capacity and specific activity of a chitinase from Trichoderma harzianum. FEMS Microbiol Lett 198:57–63
Limon MC, Chacon MR, Mejias R, Delgado-Jarana J et al (2004) Increased antifungal and chitinase specific activities of Trichoderma harzianum CECT 2413 by addition of a cellulose binding domain. Appl Microbiol Biotechnol 64:675–685
Livak KJ, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Matarese F, Sarrocco S, Gruber S, Seidl SV, Vannacci G (2012) Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 158:98–106
Migheli Q, González-Candelas L, Dealessi L et al (1998) Transformants of Trichoderma longibrachiatum overexpressing the β-1,4-endoglucanase gene egl1 show enhanced biocontrol of Pythium ultimun on cucumber. Phytopathology 88:673–677
Penttilä M, Nevalainen H, Rättö M, Salminen E, Knowles J (1987) A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61:155–164
Pillai CKS, Paul W, Chandra PS (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678
Reissig JL, Strominger JL, Leloir L (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 217:959–966
Reithner B, Ibarra-Laclette E, Mach RL, Estrella H (2011) A identification of mycoparasitism-related genes in Trichoderma atroviride. Appl Environ Microbiol 77:4361–4370
Ryder LS, Harris BD, Soanes DM, Kershaw MJ et al (2012) Saprotrophic competitiveness and biocontrol fitness of a genetically modified strain of the plant-growth-promoting fungus Trichoderma hamatum GD12. Microbiology 158:84–97
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Woodburry
Sundar AR, Das ND, Krishnaveni D (1995) In-vitro antagonism of Trichoderma spp. against two fungal pathogens of castor. Indian J Plant Protec 23:152–155
Van Aalten DMF, Komander D, Synstad B, Gaseidnes S et al (2001) Structural insights into the catalytic mechanism of a family 18 exo-chitinase. PNAS 98:8979–8984
Van de Velde K, Kiekens P (2004) Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13C NMR. Carbohydr Polym 58:409–416
Yanai K, Takaya N, Kojima N, Horiuchi H et al (1992) Purification of two chitinases from Rhizopus oligosporus and isolation and sequencing of the encoding genes. J Bacteriol 174:7398–7406
Acknowledgments
We thank Prof. Dr. R. L. Mach and Prof. Dr. M. J. Hynes for kindly providing plasmids. We wish to thank Dr. M. C. Limon for her advises. This project was supported by the National Institute of Genetic Engineering and Biotechnology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kowsari, M., Motallebi, M. & Zamani, M. Protein Engineering of Chit42 Towards Improvement of Chitinase and Antifungal Activities. Curr Microbiol 68, 495–502 (2014). https://doi.org/10.1007/s00284-013-0494-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-013-0494-3