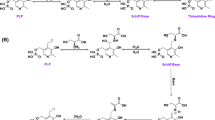
Abstract
O-acetylserine sulfhydrylase (OASS) is a key enzyme involved in the pathway of the cysteine biosynthesis. The gene of OASS from Acidithiobacillus ferrooxidans ATCC 23270 was cloned and expressed in E. coli, the soluble protein was purified by one-step affinity chromatography to apparent homogeneity. Colors and UV–vis scanning results of the recombinant protein confirmed that it was a pyridoxal 5′-phosphate (PLP)-containing protein. Sequence alignment and site-directed mutation of the enzyme revealed that the cofactor PLP is covalently bound in Schiff base linkage with K30, as well as the two residues H150 and H168 were the crucial residues for PLP binding and stabilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acidithiobacillus ferrooxidans is one of the most studied bacteria that thrives in acidic mine drainage, which obtains all its energy and electron requirements from the oxidation of various forms of reduced sulfur and ferrous iron. However, how it uses to take up sulfur(sulfate) from its environment and how it is subsequently assimilated into organic compounds such as the amino acids methionine and cysteine, the mechanisms are not clear so far [1].
O-acetylserine sulfhdrylase (OASS) is a key enzyme involved in the pathway of the cysteine biosynthesis, which is thought to be involved in the global regulation of S-assimilation, as the substrates and products of OASS have been implicated in the regulation of gene expression of the sulfate transporter [2]. It is, therefore, of interest to investigate and identify the structural features that might contribute to OASS’ unique functional properties.
The crystal structure of OASS from Salmonella typhimurium revealed that the enzyme was similar in structure to other OASS [3–5]. The active-site residues of OASS-A with the cofactor PLP covalently bound in Schiff base linkage with lysine residues. The phosphate group of the cofactor is bound at the N-terminus of helix 7, which interacts with the positive end of its dipole with the negative charge of the phosphate group. K41, k42, or k44 residues for OASS from other sources were bound to cofactor PLP to form Schiff base [6–8], but there are no reports for OASS from A. ferrooxidans so far.
In this study, the gene of OASS from A. ferrooxidans ATCC 23270 was cloned and successfully expressed in E. coli, finally purified by one-step affinity chromatography. As we know so far, this is the first report of expression in E. coli of OASS from A. ferrooxidan.
Materials and Methods
Materials
Acidithiobacillus ferrooxidans ATCC 23270 was obtained from the American Type Culture Collection. A HiTrap chelating metal affinity column was purchased from GE healthcare LTD. DH5-alpha competent cells, E. coli strain BL21 (DE3) competent cells were purchased from Invitrogen Life Technologies. The Plasmid Mini kit was purchased from Ambiogen Life Science Technology LTD. A gel extraction kit was obtained from OMEGA. Taq DNA polymerase, T4 DNA ligase, and restriction enzymes were purchased from MBI Fermentas of Germany. All other reagents were of research grade or better and were obtained from commercial sources.
Cloning of the OASS Gene from A. ferrooxidans ATCC 23270
This genomic DNA from A. ferrooxidans was used as a template for PCR reaction. The gene was amplified by PCR using primers that were designed to add six continuous histidine codons to the 5′ primer. The sequence of the forward primer was 5′-CGCGCGGATCCAGGAGGAATTTAAAATGAGAGGATCGCATCACCATCACCATCACGGTGGCTGGAGAACTGGGTGGGCAATACC-3′, containing a BamHI site (GGATCC), a ribosome binding site (AGGAGGA), codons for the amino acid sequence MRGSHHHHHH (start codon and hexahistag), and codons for amino acids 2–10 of mature OASS. The sequence of the reverse primer was 5′-CTGCAGGTCGACTTAGGCCGGAAAAACGCCCGTAGAGAGGTAGCG-3′, containing a SalI site (GTCGAC), a stop anticodon (TTA), and anticodons for amino acids 2–11 of mature cysM. PCR amplification was performed using Taq DNA polymerase, and samples were subjected to 30 cycles of 45 s of denaturation at 95°C, 45 s of annealing at 62°C, and 2 min of elongation at 72°C in a Mastercycler Personal of Eppendorf Model made in Germany. The amplification products were analyzed by electrophoresis on a 1.0% agarose gel and stained with ethidium bromide. The resulting PCR product was gel purified, double digested, and ligated into a pLM1 [9], expression vector resulting in the pLM1::cysM plasmid, then transformed E. coli strain BL21(DE3) competent cells for expression purposes.
Construction of A. ferrooxidans OASS Mutant Plasmids
A QuikChange mutagenesis kit (Stratagene) was applied for constructing the mutant expression plasmids. The plasmid pLM1::cysM was used as a template for constructing mutant expression plasmids through PCR. The following primer and their antisense primer were synthesized to introduce the mutated sequence: K30A: 5′ TGCGGATTTTCGGAGCTCTGGAGGGGAACAA-3′, codon AAG for lysine (K) was changed to codon GCT for alanine (A); K41A: 5′ CCGCCGGTTCGGTCGCTGATCGTCCCGCCTT-3′, codon AAG for lysine (K) was changed to codon GCT for alanine (A); H150A: 5′ ATAATCCCGAGGCAGCTTACCTCGGGACCGG-3′, codon CAT for histidine (H) was changed to codon GCT for alanine (A); H168A: 5′ GCGGTACCGTGACCGCTTTCGTATCGTCCAT-3′, codon CAT for histidine (H) was changed to codon GCT for alanine (A).
PCR amplification was performed using Pfu DNA polymerase and samples were subjected to 13 cycles of 0.5 min of denaturation at 95°C, 1 min of annealing at 61°C, and 12 min of elongation at 72°C in a Mastercycler Personal of Eppendorf Model made in Germany. DpnI restriction enzyme was used to digest the parental supercoiled double-stranded DNA. The isolated mutant plasmids were then used to transform E. coli strain BL21(DE3) competent cells for expression purposes.
Expression and Purification of Recombinant OASS Wild-Type and Its Mutant Proteins in E. coli
E. coli strain BL21(DE3) cells with pLM1::cysM plasmid and the mutant plasmids were grown at 37°C in 500 ml of LB medium containing ampicillin (100 mg/l) to an OD600 of 0.6. Cells were incubated at room temperature with the addition of 0.5 mM IPTG overnight with shaking at 180 rpm. The cells were harvested by centrifugation and the cell pellet was washed with an equal volume of sterile water. The cells were again harvested by centrifugation, suspended in start buffer (20 mM potassium phosphate, pH 7.4, 0.5 M NaCl), incubated with 5 mg lysozyme at room temperature for half an hour, and then stored at −80°C for purification. The method of Bradford was used to determine the protein content with bovine serum albumin as the standard. The eluted fractions were analyzed by SDS–PAGE with 18% (v/v) acrylamide.
O-Acetylserine Sulfhdrylase (OASS) Activity Assays
The activity of OASS was determined according to the method of Demosthenis Chronis [10]. One unit of enzyme activity is defined as the conversion of 1 nM of OASs into cysteine per minute under the stated assay conditions. Assays were performed three times and each time was represented by two replications.
UV-Scanning and Fluorescence of the OASS
UV–visible spectra and fluorescence were separately obtained on a Techcomp UV-2300 spectrophotometer and Hitachi FL Spectrophotometer. The temperature for measurement was maintained at 25°C.
Results and Discussion
Cloning of OASS Gene from A. ferrooxidans
PCR techniques were used to successfully add six continuous histidine residues to the N-terminal of the OASS from A. ferrooxidans. The transformant was grown in LB medium and the corresponding plasmid was isolated and re-transformed to E. coli BL21 (DE3) for expression. Mutant expression plasmids pcysM (K30A), pcysM (K41A), pcysM (H150A), and pcysM (H168A) were constructed, and their sequences were examined for the presence of directed mutations and the absence of PCR-generated random mutations by DNA sequencing.
Expression and Purification of OASS from A. ferroodoxins
The expressions of OASS from A. ferrooxidans and its mutant proteins in E. coli BL21 (DE3) were optimal with 0.5 mM IPTG concentrations at 25°C, nickel metal-affinity resin columns were used for single-step purifications of His-tagged A. ferrooxidans OASS wild-type and mutant proteins. The protein fractions were dialyzed against a 20 mM potassium phosphate buffer, pH 7.5, 5% glycerol, and 5 mM β-mercaptoethanol right after purification. The addition of 5% glycerol and 5 mM β-mercaptoethanol was essential for maintaining the long-term stability of the protein. The purified enzyme was observed to take on light-yellow color. The purity of the enzymes wild-type and mutant was examined by SDS–PAGE and single bands corresponding to the 32 kDa proteins were observed with >95% purity (Fig. 1), which was in agreement with the deduced molecular mass of OASS.
Coomassie blued-stained SDS–PAGE of the purified OASS from A. ferrooxidans and its mutant proteins. Lane 1 molecular mass standards, lane 2 purified OASS wild-type, lane 3 purified OASS mutant K30A, lane 4 purified OASS mutant K41A, lane 5 purified OASS mutant H150A, lane 6 purified OASS mutant H168A
UV-Scanning and Fluorescence of OASS
The UV–visible spectra of the recombinant OASS is shown in Fig. 2. It is very similar in spectrum the enzyme from other sources [11, 12], with an absorption band centered at 412 nm. This is indicative of a PLP molecule in an internal aldimine linkage with the active site lysine residue. Emission spectrum upon excitation at 298 nm and at 412 nm, which is shown in Fig. 3, with the direct emission at 344 nm of the aromatic residues and a longer wavelength emission at 509 nm [13–15], which was attributed to energy transfer from tryptophan to the enolimine tautomer of the internal aldimine.
Role of Conserved Residues of OASS in PLP Binding
Sequence alignment for OASS from various sources showed that the four residues of K30, K41, H150, and H168 were highly conserved (Fig. 4), which was proposed to be important for the PLP cofactor binding and stabilization. To confirm this, mutations for these four residues of OASS from A. ferroxidans were created, K30A, K41A, H150A, and H168A mutant plasmids were expressed in E. coli and then purified by affinity chromatography to homogeneity. Except K41A, the other three mutant proteins are colorless after purification, and UV–Vis scanning of the mutant proteins showed that there were no absorptions between 300 and 500 nm (Fig. 5). The wild-type OASS has an enzyme activity of 5.0 × 104 U/mg, while the mutant proteins do not have. The results strongly suggested that the active-site residues of OASS and the cofactor PLP covalently bound in Schiff base linkage with K30, as well as the two residues H150A and H168A were crucial residues for PLP binding and stabilization.
The sequence alignment of OASS from A. ferrooxidans and other sources. A. ferrooxidans from A. ferrooxidans ATCC 23270; H. arsenicoxydans from Herminiimonas arsenicoxydans; A. vinosum from Allochromatium vinosum; H. halophila SL1 from Halorhodospira halophila SL1; B. cepacia from Burkholderia cepacia. The four residues proposed to coordinate a PLP cofactor are marked in asterisk. The highly conserved residues are marked in black
Summary
We reported here the first expression in E. coli of His-tagged cysM from A. ferrooxidans. The OASS was a PLP sequence dependant type enzyme. Sequence alignment and site-directed mutation of the enzyme revealed that K30, H150 and H168 were crucial residues for PLP binding. Analyses of the enzyme activity indicate that OASS can catalyze sulfide and OAS formation of cysteine.
References
Rawlings DE (2001) The molecular genetics of Thiobacillus ferrooxidans and other mesophilic, acidophilic, chemolithotrophic, iron- or sulfur-oxidizing bacteria. Hydrometallurgy 59:187–201
Valdés J, Veloso F, Eugenia J, Holmes D (2003) Metabolic reconstruction of sulfur assimilation in the extremophile Acidithiobacillus ferrooxidans based on genome analysis. BMC Genomics 4(51):1471–2164
Burkard P, Jagannatha Rao GS, Hohenester E et al (1998) Three-dimensional structure of O-acetylserine sulfhydrylase from Salmonella typhimurium. J Mol Biol 283:121–133
Jansonius JN, Vincent MG (1987) Structural basis for catalysis by aspartate aminotransferase. Biol Macromol Assemblies 187–288
Rao GS, Mottonen J, Goldsmith EJ (1993) Crystallization and preliminary X-ray data for the A-isozyme of O-acetylserine sulfhydrylase from Salmonella typhimurium. J Mol Biol 231:1130–1132
Hyde CC, Ahmed SA, Padlan EA et al (1988) Three-dimensional structure of the tryptophan synthase α2β2 multienzyme complex from Salmonella typhimurium. J Biol Chem 263:17857–17871
Rege VD, Kredich NM, Tai CH et al (1996) A change in the internal aldimine lysine(K42) in O-acetylserine sulfhydrylase to alanine indicates its importance in transamination and as general base catalyst. Biochemistry 35:13485–13493
Schnell R, Oehlmann W, Singh M et al (2007) Structural insights into catalysis and inhibition of O-acetylserine sulfhydrylase from Mycobacterium tuberculosis. J Biol Chem 282:23473–23481
Sodeoka M, Larson C, Chen L, Land W, Verdine G (1993) A multifunctional plasmid for protein expression by ECPCR: overproduction of the p50 subunit of NF-KB. Bioorg Med Chem Lett 3:1095–1100
Warrilow A, Hawkesford M (1998) Separation, subcellular location and influence of sulphur nutrition isoforms of cysteine synthase in spinach. J Exp Bot 49: 327,625–1636
Becker MA, Tomkins GM (1969) Pleiotropy in a cysteine-requiring mutant of Salmonella typhimurium resulting from altered protein–protein interaction. J Biol Chem 244:6023–6030
Cook PF, Wedding RT (1976) A reaction mechanism from steady state kinetic studies for O-acetylserine sulfhydrylase from Salmonella typhimurium LT-2. J Biol Chem 251:2023–2029
Benci S, Vaccari S, Mozzarelli A, Cook PF (1999) Time-resolved fluorescence of O-acetylserine sulfhydrylase. Biochim Biophys Acta 1429:317–330
McClure GD Jr, Cook PF (1994) Product binding to the alphacarboxyl subsite results in a conformational change at the active site of O-acetylserine sulfhydrylase-A: evidence from fluorescence spectroscopy. Biochemistry 33:1674–1683
Strambini GB, Cioni P, Cook PF (1996) Tryptophan luminescence as a probe of enzyme conformation along the O-acetylserine sulfhydrylase reaction pathway. Biochemistry 35:8392–8400
Acknowledgments
This work was supported by National Excellent Ph D Theses of P R China (Grant No.200549), National Natural Science Foundation of P. R. China (Grant No.50874032, No. 50374075) and the shanghai Leading Academic Discipline Project(Grant No.B604).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zheng, C., Nie, L., Qian, L. et al. K30, H150, and H168 Are Essential Residues for Coordinating Pyridoxal 5′-Phosphate of O-Acetylserine Sulfhydrylase from Acidithiobacillus ferrooxidans . Curr Microbiol 60, 461–465 (2010). https://doi.org/10.1007/s00284-009-9565-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9565-x