Abstract
Purpose
Methotrexate (MTX) induces hepatotoxicity, limiting its clinical efficacy as a widely known chemotherapy drug. In the current study, we examined the protective effect of human placenta extract (HPE) against MTX-induced liver damage in rats, as well as its ability to regulate antioxidative and anti-inflammatory liver responses.
Methods
Male rats were orally administered MTX at a daily dose of 5 mg/kg-body-weight in the presence or absence of HPE (10.08 mg/kg) for 2 weeks. We measured the biological effects of MTX and HPE on the levels of liver enzymes, lipid profile, lipid peroxidation, oxidative stress biomarkers, and cytokines [tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10)]. In addition, histological examination and histopathological scoring of liver tissues were performed.
Results
MTX-treated rats showed significantly increased (p < 0.001) liver enzyme levels for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, total cholesterol, and triglyceride levels. However, HPE supplementation in MTX-treated rats significantly decreased (p < 0.001) these elevated levels. HPE supplementation also significantly reduced the oxidative stress biomarker malondialdehyde (MDA), reversed the reduction in glutathione (GSH), and markedly increased the antioxidant enzyme activities of catalase (CAT) and superoxide dismutase (SOD) in the livers of MTX-treated rats. Furthermore, HPE supplementation significantly decreased the MTX-elevated levels of the pro-inflammatory cytokines TNF-α, IL-6, and IL-10. Histopathological examinations showed that MTX produced severe cellular damage and inflammatory lesions in liver tissues, while treatment with HPE improved hepatic histologic architecture.
Conclusion
HPE has the ability to ameliorate methotrexate-induced liver injury in rats by mechanisms that include boosting antioxidative responses and down-regulating MDA and pro-inflammatory cytokine production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methotrexate (MTX), a dihydrofolate reductase inhibitor, is an anti-metabolite used as a chemotherapeutic agent against different types of malignancies [1,2,3]. MTX is also used to treat a variety of other diseases, including autoimmune diseases, such as rheumatoid arthritis, psoriasis, inflammatory bowel disease, and vasculitis [4,5,6,7]. However, MTX induces neurotoxicity [8,9,10,11], nephrotoxicity [12], and hepatotoxicity [13, 14], with MTX being linked to a raised hazard of liver damage, cirrhosis, and fibrosis. MTX acts as a dihydrofolic acid analog that binds to the dihydrofolic acid reductase enzyme by inhibiting the synthesis of tetrahydrofolate, which is required for DNA synthesis. Administration of MTX results in an excess output of reactive oxygen species (ROS), which excite many of the pathological processes [15, 16].
Many risk factors can increase the hepatotoxic effect of MTX, including age, duration of exposure to MTX and its cumulative dose, alcohol consumption, history of non-alcoholic steatohepatitis, diabetes, obesity, hepatitis B or C virus infection, and hepatotoxic drugs [17, 18]. Furthermore, non-alcoholic fatty liver disease, in which hepatic steatosis is observed without heavy alcohol consumption [19], has also been linked to MTX therapy [20, 21]. As a serious side effect even at low doses, MTX can lead to hepatic fibrosis and cirrhosis. Low-dose MTX use in psoriasis was found to have a 7% risk of cirrhosis development, and transaminase elevations up to three times normal were seen in 8% of the patients monitored [22]. In addition, Sakthiswary et al. reported that the cumulative dose of MTX was found to have a significant positive correlation with the alanine transaminase (ALT) level [23].
Recent studies have reported the effectiveness of several agents against MTX-induced liver damage, including casticin, myricetin [24], and berberine [11], a natural isoquinoline alkaloid that can be isolated from Coptis chinensis. In the current study, we examined the protective effect of human placenta extract (HPE) against MTX-induced hepatotoxicity in rats. The placenta is a unique organ, connecting the wall of a uterus to a growing fetus. The placenta allows the developing fetus to intake nutrients, eliminate waste, exchange gases, and thermo-regulate through the mother’s blood. This organ interchanges substances between the maternal and fetal blood streams without mixing the two streams or allowing corporeal contact between them [25]. It has become evident that human placenta is the source of a large number of biologically active molecules [26], such as hepatocyte growth factor (HGF) [27], epidermal growth factor (EGF) [28], and transforming growth factor-a (TGF-a) [29]. HPE also contains l-tryptophan as a main antioxidant constituent [30]. In addition, HPE has been used to provide multiple beneficial therapeutic effects, including wound healing [31], health status improvement in elderly subjects [32], and stress reduction [33]. HPE was also reported to improve hepatic damage via liver regeneration [34] and to decrease inflammatory response and apoptosis [35]. These results motivated the present study which aimed to evaluate the protective effects of HPE on MTX-induced hepatotoxicity and to investigate its regulatory antioxidative and anti-inflammatory effects on the liver of rats.
Materials and methods
Animals
Forty male albino rats of the Wistar strain (Rattus norvegicus) were used in this study. Rats 3 months old (weighing 150 ± 4 g) were purchased from the Laboratory Animal Breeding Colony of the National Research Centre, Dokki Giza, Egypt. Rats were allowed to acclimate for 1 week before the study’s start. Rats were housed in plastic cages under natural room temperature (26 ± 2 °C) with a relative humidity of 50 ± 5% and 12 h dark/light cycle. Food and water were available ad libitum. Food consisted of pellets that were 54% carbohydrate, 3% fat, 26% protein, and 17% vitamins and minerals.
Drugs and chemicals
MTX ampoules were administered as the clinical formulation. MTX was manufactured by the Hikma Specialized Pharmaceutical Company, Cairo, Egypt. HPE is commercially known as Laennec. Laennec was obtained from YHB Pharma Company, Cairo, Egypt. Each ampoule is water-soluble and consists of a 2 ml solution containing 112 mg of human placenta. All other chemicals used were analytical grade.
Study design
Rats were randomly divided into four groups with ten animals per group as follows: Group 1 (control): untreated; Group 2 (MTX): injected intraperitoneally (i.p.) with MTX at a dose level of 5 mg/kg-body-weight in physiological saline for 5 days according to [36]; Group 3 (HPE): injected subcutaneously with HPE at a dosage of 10.08 mg/kg-body-weight (equivalent to the therapeutic dose for humans) for 2 weeks [37]; Group 4 (MTX plus HPE): treated with MTX at the same dose and duration as Group 2 and simultaneously treated with HPE at the same dose and duration as Group 3.
At the end of the experimental period, all rats were anesthetized by intravenously administering an overdose of 30–50 mg/kg pentobarbital and then sacrificed by cervical dislocation. Liver tissues were quickly removed and washed using an ice-cold saline solution, then they were cut into small pieces. The first part for homogenates was homogenized in potassium phosphate buffered (pH 7.4), then centrifuged to obtain the supernatants which were kept at -800C until analyzed. The second part of liver tissues were excised and fixed in 10% neutral formalin solution for histopathological analysis.
Biochemical analyses
Liver function
The activities of both AST and ALT and were measured using colorimetric assay of Gella et al. [38]. Alkaline phosphatase (ALP) was assayed according to the method of Kind and King [39]. Serum total bilirubin was determined according to Kaplan et al. [40]. Total cholesterol (TC) and triglyceride (TG) levels were analyzed using diagnostic kits according to Burtis et al. [41].
Liver lipid peroxidation
Liver tissue homogenates were analyzed using the spectrophotometric method according to the manufacturer’s protocol of Biodiagnostic, Egypt. The malondialdehyde (MDA) content was assayed in the form of thiobarbituric acid-reactive substances (TBARS) in liver according to the method described previously in [42]. Briefly, 500 µl of sample was added to 1 ml of trichloroacetic acid (TCA 15%) and centrifuged at 3000 rpm for 10 min. 1 ml of supernatant was mixed with 500 µl of thiobarbituric acid (TBA 0.7%), heated in boiling water bath for 10 min, and cooled, and the color was read at 532 nm. The TBARS level was calculated against control according to the following equation: TBARS level (nmol/ml) = Absorbance/0.156. The majority of TBARS are MDA, and thus the concentration of MDA in the sample homogenate was expressed as nmol MDA/mg protein. The results were calculated using an index of absorption for MDA using a molar extinction coefficient 1.56 × 105/M/cm.
Endogenous antioxidant activities
Glutathione (GSH) activity was assayed as described in [43]. Briefly, 100 µl sample (test), distilled water (Blank), and GSH (standard) were mixed with 100 µl of sulphosalicylic acid (4%), kept at 4 °C for at least 1 h, and then centrifuged at 1200 g for 10 min at 4 °C. 100 µl supernatant was then mixed with 2.7 ml phosphate buffer (0.1 M, pH 7.4) and 0.2 ml DTNB [5,5ʹ-dithiobis-(2-nitrobenzoic acid)] and incubated for 5 min. Measurements of the resulting yellow color were immediately conducted at 412 nm. A standard curve was constructed using standard GSH. Finally, GSH content was expressed as mg/mg protein.
The levels of superoxide dismutase (SOD) activity was evaluated as described in [44]. In the assay of SOD, 20 µl of sample (test) or buffer and 10 µl of pyrogallol (20 mM in 10 mM HCl) were added to 1 ml buffer solution. The sample absorbances for test (At) and reference (Ar) were measured against air after 30 min and 90 min at 420 nm. The percentage by which pyrogallol autoxidation was inhibited was calculated via: [% inhibition] = [100 – (At/min/ml sample)/(Ar/min/ml)] × 100. Using the SOD standard curve, one unit was found to equal 153 ng, so sample enzyme activity [U/mg protein] was calculated as specific activity divided by 153.
The levels of catalase (CAT) were assayed according to the method described in [45]. In the sample cuvette, 0.1 ml of sample was mixed with 0.5 ml of 0.2 M sodium phosphate buffer at pH 7.6 and 0.3 ml of 0.5% H2O2. The mixture was brought to a final volume of 3 ml with distilled water. The breakdown of H2O2 was recorded by measuring the absorbance at 240 nm and the enzyme activity was calculated as the change in absorbance per minute.
Cytokine expression
Tumor necrosis factor-alpha (TNF-α) levels were estimated using ELISA-based cytokine kits of Global Headquarters according to [46]. Interleukin-6 (IL-6) was measured using MyBioSource ELISA kits as described in [47], and IL-10 was assayed using ThermoFisher ELISA kits according to [48].
Histopathology
Fresh liver tissues from each group were fixed in 10% neutral formalin at room temperature for 24 h, dehydrated in ascending grades of ethanol, and embedded in paraffin. Tissue blocks were cut at 4 μm thick. Paraffin sections were stained with hematoxylin and eosin dye and examined using a digital light microscope (Olympus, Tokyo, Japan). The histological scoring was assessed according to López-Alonso [49]. A minimum of 3 slides for each animal and 10 fields per slide were examined and scored semi-quantitatively for the severity of changes. The scoring was done as none (−), mild (+), moderate (+ +), and severe (+ + +) changes.
Statistical analysis
Data analysis was performed using SPSS v20.0 (Armonk, NY: IBM Corp). The Kolmogorov–Smirnov test was used to verify the normality of distribution. Quantitative data were described using range (minimum and maximum), mean, and standard deviation (SD). The used tests were F test (ANOVA) for normally distributed quantitative variables to compare between more than two groups, and Post Hoc Tukey test for pairwise comparisons. p values ≤ 0.05 were considered statistically significant.
Results
HPE regulates serum liver function
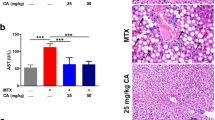
Data in Fig. 1 show the serum levels of AST, ALT, and ALP of all experimental groups. There were insignificant differences in the levels of AST, ALT, and ALP between the control group and HPE treated group. MTX-treated rats showed a highly significant increase (p < 0.001) in AST (82.5 ± 3.98), ALT (69.1 ± 1.38), and ALP (203.09 ± 5.43) levels compared with the control group. However, supplementation with both MTX and HPE resulted in a significant decrease in AST (59.75 ± 0.6), ALT (52.55 ± 1.64), and ALP (149.62 ± 1.05) levels relative to the MTX-treated group (p < 0.001), although a significant increase (p < 0.05) was still recorded in comparison with the control group.
Effect of MTX and HPE administration on serum liver function for A AST, B ALT, and C ALP activity. Each value represents the mean ± SD for 10 animals per group. ***Highly significant in comparison with the control group at p < 0.001. *Significant in comparison with the control group at p < 0.05. aHighly significant in comparison with the MTX group at p < 0.001
Lipid levels
Figure 2 shows the lipid profile levels of serum total bilirubin levels, total cholesterol, and triglycerides. Results exhibited insignificant differences between the control group and HPE treated group. When rats were treated with MTX, a highly significant rise (p < 0.001) in total bilirubin (1.01 ± 0.03), cholesterol (180.5 ± 2.59), and triglyceride (194 ± 6.92) levels were represented as compared with that of control group. On the other hand, we noted again that the combining effect of MTX and HPE resulted in highly significant (p < 0.001) reduction in the levels of total bilirubin (0.43 ± 0.02), cholesterol (143.5 ± 2.59), and triglycerides (163.5 ± 4.33) compared with the MTX group, although a significant increase (p < 0.01) was still recorded in comparison with the control group.
Effect of MTX and HPE administration on lipid levels of the different experimental groups for A total bilirubin, B cholesterol, and C triglycerides. Each value represents the mean ± SD for 10 animals per group. ***Highly significant in comparison with the control group at p < 0.001. **Significant in comparison with the control group at p < 0.01. aHighly significant in comparison with the MTX group at p < 0.001. bSignificant in comparison with the MTX group at p < 0.01
HPE regulates lipid peroxidation and antioxidant activities
The liver lipid peroxidation (MDA) and antioxidant markers (GSH, SOD, and CAT) are shown in Fig. 3. Data show that liver MDA concentration (16.4 ± 1.14) in MTX-treated rats was significantly higher (p < 0.001) as compared with control rats (Fig. 3A). However, supplementation with HPE counteracted the increase in MDA related to MTX. HPE significantly reduced the mean MDA values (11.2 ± 0.82) (p < 0.001). The effect of HPE administration on GSH levels was also examined in the liver of different groups. MTX-treated rats had a highly significant depletion (p < 0.001) in comparison with the control group (Fig. 3B), but HPE treatment significantly increased the GSH levels with value (7.38 ± 0.26). MTX administration significantly decreased the antioxidant enzyme activities of CAT (45.6 ± 0.35) (Fig. 3C) and SOD (4.48 ± 0.91) (Fig. 3D) in liver tissues in comparison with the control group, but data show a marked increase (p < 0.001) in tissue concentrations of CAT (85.4 ± 6.26) and SOD (7.94 ± 0.69) activity in liver tissues post exposure to HPE as compared with the MTX group.
Effect of MTX and HPE administration on lipid peroxidation and antioxidant activities for A MDA, B GSH, C SOD, and D CAT. Each value represents the mean ± SD for 10 animals per group. ***Highly significant in comparison with the control group at p < 0.001. *Significant in comparison with the control group at p < 0.05. aHighly significant in comparison with the MTX group at p < 0.001
HPE regulates the expression of pro-inflammatory cytokines TNF-α, IL-6, and IL-10
Regarding pro- and anti-inflammatory markers, MTX injection caused significantly increased expression (p < 0.001) of TNF-α, IL-6, and IL-10 with values (16.54 ± 0.76, 129.4 ± 3.64 and 17.5 ± 1.25), respectively, in the liver tissues (Fig. 4). On the other hand, rats treated with HPE succeeded in counterbalancing this increase and returned to normal levels when compared to the MTX group.
Effect of MTX and HPE administration on the expression of pro-inflammatory cytokines A TNF-α, B IL-6, and C IL-10. Each value represents the mean ± SD for 10 animals per group. ***Highly significant in comparison with the control group at p < 0.001. *Significant in comparison with the control group at p < 0.05. aHighly significant in comparison with the MTX group at p < 0.001
HPE protects hepatocytes against MTX‑induced hepatotoxicity
Hematoxylin and eosin (H&E) light micrographs of liver sections of rats are illustrated in Fig. 5. Photomicrographs from the control group show normal liver tissue architecture (Fig. 5A). The normal control structural unit of the liver is the hepatic lobule which appears roughly hexagonal in shape and is centered by a thin-walled vessel. Hepatocytes appear as large polygonal cells with round prominent nuclei. The space between these lobules contains capillaries and the liver sinusoids; these are irregularly dilated vessels in intimate contact with the hepatocytes. These sinusoids are lined by flat endothelial cells which could be readily distinguishable from hepatocytes by their flattened condensed nuclei and poorly stained cytoplasm. On the other hand, liver sections of MTX-treated rats revealed hepatocellular injury in some areas, as represented by the loss of the normal architecture of the liver tissues. Cytoplasmic vacuolation is evident. In addition, sinusoidal dilations and hyperemia (blood congestion) in sinusoids and central veins can be seen in Fig. 5B, as well as diffused and periportal leucocytic infiltration and increased numbers of Kupffer cells in Fig. 5C, D. The light microscopic examination of the liver tissues from rats treated with only HPE were found to be comparable to that of the control rats (Fig. 5E). However, screening of liver sections of rats treated with both MTX plus HPE showed minimal morphological changes in the histological features all over the liver tissue, with the hepatocytes appearing as typically polygonal with distinct cell margins and well-defined cytoplasm. The nuclei of the hepatocytes appeared to be normal. These results indicate an obvious protective role of HPE against the action of MTX (Fig. 5F). The histological scoring was graded and results are summarized in Table 1.
Effects of HPE treatment on histopathological injury in liver tissues following MTX-induced hepatotoxicity. A Control group showing normal histological architecture of the hepatocytes (HC); central vein (CV); and Kupffer cell (Kc). B, C, D MTX-treated group showing hepatocytes (HC) with cytoplasmic vacuolization (thin arrow); dilation for both central vein (CV) and portal vein (PV); inflammatory leucocytic infiltrations (thick arrow); and bile ducts (Bd) hyperplasia. E Rats treated with both MTX and HPE showed hepatocytes (HC) with normal nuclei (N) and central vein (CV). F HPE-treated rats showed normal hepatocytes (HC) with round basophilic nuclei (N) and normal Kupffer cell (Kc). Original Magnification: 200x
Discussion
Methotrexate (MTX)-induced hepatotoxicity is a serious problem, because it affects MTX’s clinical therapeutic effects. Oxidative stress and lipid peroxidation mediated by oxygen free radicals have been considered as an important cause of MTX-induced neurotoxicity [8,9,10,11], hepatotoxicity [13, 14], intestinal toxicity [50], and nephrotoxicity [12]. The present study demonstrates the ability of human placenta extract (HPE) to ameliorate MTX-induced hepatotoxicity. MTX still remains one of the major causes of drug-induced steatohepatitis. Earlier studies investigated the cumulative dose of MTX-associated nonalcoholic fatty liver disease (NAFLD) with transaminitis, and it was found to have a significant positive correlation with the ALT level [23]. MTX reduces oxygen uptake and decreases oxidative phosphorylation in isolated mitochondria [51]; in addition, it inhibits several mitochondrial enzymes including 2-oxoglutarate, isocitrate, malate, and pyruvate dehydrogenases [52]. Treatment with HPE significantly reduced the biochemical and histological alterations induced by MTX in the liver of rats by regulating antioxidative responses and down-regulating MDA.
In the current study, MTX-treated rats demonstrated significantly increased levels of lipid peroxidation in the liver as exemplified by a significant increase in level of malondialdehyde (MDA) and a decrease in the antioxidants glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD). These results are in accordance with others [25, 41, 42, 53,54,55]. It is of great interest to note that HPE supplementation resulted in a significant protective effect against MTX-induced lipid peroxidation. HPE reduced the level of MDA, reversed the reduction in GSH, and markedly increased the antioxidant enzyme activities of CAT and SOD in the livers of MTX-treated rats. Several studies on the anti-inflammatory and anti-oxidative activities of HPE have been conducted in different models, including porcine placenta extracts against contact dermatitis in vivo [56], benzo[a]pyrene-exposed rats [57] and concanavalin A-induced liver injury in mice [58]. In addition, other studies have shown that berberine, a natural isoquinoline alkaloid, can mitigate MTX-induced oxidative stress and inflammation in the liver tissue [59]. Excessive ROS generation by MTX can downregulate the antioxidant defense mechanism in the liver. Earlier studies showed exposure to MTX leads to upregulation of nuclear translocation of NF-κB and phosphorylated Iκ-B, down-regulation of antiapoptotic protein Bcl-2, and increased levels of caspase 3 that subsequently lead to cell death [60].
GSH is a protective endogenous antioxidant that plays an essential role in combatting free radicals and other oxidants. Our prior work [61] and that of others observed depletion of GSH levels in aged mice [62]. The current study shows that MTX-treated rats have significantly lower levels of antioxidant enzymes (GSH, CAT, and SOD) in comparison with the control group. Enzyme activation induced by HPE may increase the hydrogen peroxide to water conversion rate mediated by GPx and CAT as well as the superoxide to hydrogen peroxide conversion rate mediated by SOD [63, 64]. HPE treatment provided antioxidant effects not only on the non-enzymatic defense system (GSH) but also on the antioxidant enzyme activities of SOD and CAT, which have the capacity to neutralize free radicals and reduce cellular death and disease development. Thus, the use of HPE gives protection against various free radicals by inhibiting hydroxyl radicals and superoxide anions and by decreasing the lipid peroxidation level.
The correlation between MTX-related changes in lipid metabolism and oxidative stress has been of great interest. Earlier studies show how changes to the lipid profile result in increased oxidative stress. Increased lipid content is thought to result in decreased antioxidant enzyme expression, increased NADPH oxidase expression, and increased ROS concentrations [65]. Results in Figs. 1 and 2 show that MTX significantly altered the oxidant/antioxidant balance that caused severe liver injury, manifested as augmented activities of ALT, AST, ALP, serum total bilirubin levels, cholesterol, and triglycerides levels. These results are in accordance with others [66, 67]. HPE supplementation in MTX-treated rats resulted in a significant decrease in ALT, AST, and ALP activities relative to the MTX group. Furthermore, HPE-treated rats exhibited significant decreases in the concentrations of total bilirubin, total cholesterol, and triglycerides relative to the MTX group, suggesting that HPE may reduce oxidative stress by changing the lipid profile and indicating HPE’s ability to have a hepato-protective effect. In addition, the current study demonstrates that MTX induces pathological changes in the liver, including necrosis, inflammatory cell infiltration, hepatocyte apoptosis, degeneration, vacuolation, and bile duct hyperplasia. However, these hepatotoxic changes were significantly attenuated by HPE treatment, suggesting that HPE could effectively counteract MTX-induced liver cell injury.
The underlying mechanisms of HPE’s action is not clear, but it may involve its antioxidant and anti-inflammatory activities. HPE suppressed the expression of inflammation- and fibrosis-related genes and NADPH oxidase, especially in the perivascular regions. This suggests that reductions in oxidative stress and inflammation are key beneficial effects of HPE. These results are in accordance with [68]. Our observed results of HPE acting in concert with MTX matches closely with the recent study by Cataldi et al. of a new avenue for treatment and prevention of drug-induced steatosis and steatohepatitis [69], where they noted promising work in in vitro and animal studies of combining a hepatotoxic drug with a second molecule that aimed to antagonize liver toxicity as well as enhance the pharmacological activity of the drug. We do also note that while the present study reports on HPE’s antioxidant and anti-inflammatory activities, it is possible that HPE could potentially modify liver fat accumulation. This represents a main limitation of the current study and should be studied in the future.
In the current study, the MTX-treated group showed increased levels of TNF-α, IL-6, and IL-10 cytokines in the hepatic tissue, in agreement with others [53, 54]. This effect can be explained by [57, 67], who concluded that MTX-induced oxidative stress and lipid peroxidation caused degradation of IκB proteins and released NF-κB p65, the active subunit of NF-κB, in the cytoplasm. Translocation of NF-κB p65 to the nucleus promotes gene transcription of inflammatory cytokines. Our results showed that HPE supplementation decreased the production of TNF-α, IL-6, and IL-10, cytokines that play a central role in mediating inflammatory response. The imbalance of pro-inflammatory cytokines and anti-inflammatory cytokines may represent another important mechanism of liver injury.
The ability of HPE to protect hepatocytes against MTX‑induced hepatotoxicity is well illustrated by the H&E light micrographs of rats' liver sections shown in Fig. 5. Screening of liver sections of rats treated with both MTX plus HPE showed minimal morphological changes. The hepatocytes appeared as typically polygonal with distinct cell margins and well-defined cytoplasm, and the nuclei appeared to be normal. MTX produced severe cellular damage and inflammatory lesions in liver tissues, while treatment with HPE improved hepatic histologic architecture.
In conclusion, our results show that human placental extract (HPE) treatment ameliorates MTX-induced liver toxicity in rats. HPE exerts a hepatoprotective effect by improving the activity of endogenous antioxidant enzymes, increasing lipid peroxidation, and suppressing inflammatory responses.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Ayad MW, El Naggar AA, El Naggar M (2014) MTHFR C677T polymorphism: association with lymphoid neoplasm and effect on methotrexate therapy. Eur J Haematol 93:63–69
Yu WJ et al (2020) Polymeric nanoscale drug carriers mediate the delivery of methotrexate for developing therapeutic interventions against cancer and rheumatoid arthritis. Front Oncol 10:1734
Tsukamoto S et al (2020) Current treatment considerations for osteosarcoma metastatic at presentation. Orthopedics 43:e345–e358
Sakthiswary R, Suresh E (2014) Methotrexate in systemic lupus erythematosus: a systematic review of its efficacy. Lupus 23:225–235
Roenigk HH et al (1998) Methotrexate in psoriasis: consensus conference. J Am Acad Dermatol 38:478–485
Fournier MR et al (2010) Changes in liver biochemistry during methotrexate use for inflammatory bowel disease. Am J Gastroenterol 105:1620–1626
Xu P et al (2014) The efficacy and safety of methotrexate in refractory Crohn’s disease. Zhonghua Nei Ke Za Zhi 53:188–192
Buizer AI et al (2006) Behavioral and educational limitations after chemotherapy for childhood acute lymphoblastic leukemia or Wilms tumor. Cancer 106:2067–2075
Inaba H et al (2008) Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol 19:178–184
Vezmar S et al (2009) Methotrexate-associated alterations of the folate and methyl-transfer pathway in the CSF of ALL patients with and without symptoms of neurotoxicity. Pediatr Blood Cancer 52:26–32
Mahmoud A et al (2017) Berberine mitigates methotrexate-induced oxidative stress and inflammation in the cerebrum of rats. J Appl Pharm Sci 7:43–49
Abd El-Twab SM et al (2016) 18 β-Glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren Fail 38:1516–1527
Mahmoud AM et al (2017) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: protective effect of 18β-glycyrrhetinic acid. Chem Biol Interact 270:59–72
Cheng HS, Rademaker M (2018) Monitoring methotrexate-induced liver fibrosis in patients with psoriasis: utility of transient elastography. Psoriasis (Auckl) 8:21–29
Maeda T et al (2010) Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate-treated rats. Cancer Chemother Pharmacol 65:1117–1123
Herman S, Zurgil N, Deutsch M (2005) Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res 54:273–280
Langman G, Hall PM, Todd G (2001) Role of non-alcoholic steatohepatitis in methotrexate-induced liver injury. J Gastroenterol Hepatol 16:1395–1401
Felson DT, Anderson JJ, Meenan RF (1992) Use of short-term efficacy/toxicity tradeoffs to select second-line drugs in rheumatoid arthritis. A metaanalysis of published clinical trials. Arthritis Rheum 35:1117–1125
Chalasani N et al (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55:2005–2023
Rau R, Karger T, Herborn G, Frenzel H (1989) Liver biopsy findings in patients with rheumatoid arthritis undergoing longterm treatment with methotrexate. J Rheumatol 16:489–493
Adams LA, Angulo P (2006) Treatment of non-alcoholic fatty liver disease. Postgrad Med J 82:315–322
Walker TM, Rhodes PC, Westmoreland C (2000) The differential cytotoxicity of methotrexate in rat hepatocyte monolayer and spheroid cultures. Toxicol In Vitro 14:475–485
Sakthiswary R, Chan GYL, Koh ET, Leong KP, Thong BYH (2014) Methotrexate-associated nonalcoholic fatty liver disease with transaminitis in rheumatoid arthritis. Sci World J 2014:823763
Eki̇nci̇-Akdemi̇r FN, et al (2018) The effects of casticin and myricetin on liver damage induced by methotrexate in rats. Iran J Basic Med Sci 21:1281–1288
Devi C, Raghupathy N (2013) The histological findings in human placenta at different gestational ages. IOSR J Dental Med Sci 6:29–31
Uehara Y, Kitamura N (1996) Hepatocyte growth factor/scatter factor and the placenta. Placenta 17:97–101
Saito S, Sakakura S, Enomoto M, Ichijo M, Matsumoto K, Nakamura T (1995) Hepatocyte growth factor promotes the growth of cytotrophoblasts by the paracrine mechanism. J Biochem 117:671–676
Hofmann GE, Scott RT Jr, Bergh PA, Deligdisch L (1991) Immunohistochemical localization of epidermal growth factor in human endometrium, decidua, and placenta. J Clin Endocrinol Metab 73:882–887
Lysiak JJ, Han VKM, Lala PK (1993) Localization of transforming growth factor in the human placenta and decidua: role in trophoblast growth. Biol Reprod 49:885–894
Watanabe S et al (2002) L-tryptophan as an antioxidant in human placenta extract. J Nutr Sci Vitaminol 48:36–39
Hong JW et al (2010) The effect of human placenta extract in a wound healing model. Ann Plast Surg 65:96–100
Kong M, Park SB (2012) Effect of human placental extract on health status in elderly Koreans. Evid Based Complement Alternat Med 2012:732915
Park H-J et al (2018) Anti-stress effects of human placenta extract: possible involvement of the oxidative stress system in rats. BMC Complement Altern Med 18:149
Jung J et al (2015) Epigenetic alterations of IL-6/STAT3 signaling by placental stem cells promote hepatic regeneration in a rat model with CCl4-induced liver injury. Int J Stem Cells 8:79–89
Chatterjee P et al (2016) Human placenta-derived stromal cells decrease inflammation, placental injury and blood pressure in hypertensive pregnant mice. Clin Sci (Lond) 130:513–523
Kutluana U et al (2018) Can leflunomide prevent methotrexate induced liver toxicity? Pamukkale Tıp Dergisi 11:321–327
Paget G et al (1983) Evaluation drug activities pharmacokinetics. Academic Press, New York
Gella FJ et al (1985) A simple procedure for the routine determination of aspartate aminotransferase and alanine aminotransferase with pyridoxal phosphate. Clin Chim Acta 153:241–247
Kind P, King E (1954) Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol 7:322–326
Kaplan A et al (1984) Bilirubin. Clinical chemistry: theory, analysis and correlation, 3rd edn. The CV Mosby Co St Louis, Toronto, Princeton, pp 1238–1241
Burtis CA, Ashwood ER, Bruns DE (2012) Tietz textbook of clinical chemistry and molecular diagnostics-e-book. Elsevier, Amsterdam
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Nishikimi M, Roa N, Yogi K (1972) Determination of superoxide dismutase in tissue homogenate. Biochem Bioph Res Common 46:849–854
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Salek-Ardakani S, Croft M (2010) Tumor necrosis factor receptor/tumor necrosis factor family members in antiviral CD8 T-cell immunity. J Interferon Cytokine Res 30:205–218
Hirano T (1998) Interleukin 6 and its receptor: ten years later. Int Rev Immunol 16:249–284
Moore KW et al (1990) Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 248:1230–1234
López-Alonso B et al (2019) Histopathological and ultrastructural changes after electroporation in pig liver using parallel-plate electrodes and high-performance generator. Sci Rep 9:1–12
El-Sheikh AA, Morsy MA, Hamouda AU (2016) Protective mechanisms of thymoquinone on methotrexate-induced intestinal toxicity in rats. Pharmacogn Mag 12(Suppl 1):S76-81
Yamamoto N, Oliveira MB, de Campello AP, Lopes LC, Klüppel ML (1988) Methotrexate: studies on the cellular metabolism. I. Effect on mitochondrial oxygen uptake and oxidative phosphorylation. Cell Biochem Funct 6:61–66
Caetano NN, Campello AP, Carnieri EG, Kluppel ML, Oliveira MB (1997) Effect of methotrexate (MTX) on NAD(P)+ dehydrogenases of HeLa cells: malic enzyme, 2-oxoglutarate and isocitrate dehydrogenases. Cell Biochem Funct 15:259–264
Çetin A et al (2008) Role of grape seed extract on methotrexate induced oxidative stress in rat liver. Am J Chin Med 36:861–872
Uraz S et al (2008) Role of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicity. Dig Dis Sci 53:1071–1077
Ali N et al (2017) Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: an experimental approach. Chem Biol Interact 272:80–91
Heo JH et al (2018) Topical anti-inflammatory and anti-oxidative effects of porcine placenta extracts on 2, 4-dinitrochlorobenzene-induced contact dermatitis. BMC Complement Altern Med 18:331
Park S et al (2010) Anti-oxidative and anti-inflammatory activities of placental extracts in benzo [a] pyrene-exposed rats. Placenta 31:873–879
Liu J et al (2019) The protective effect of sheep placental extract on concanavalin A-induced liver injury in mice. Molecules 24:28
Germoush MO, Mahmoud AM (2014) Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J Cancer Res Clin Oncol 140:1103–1109
Mukherjee S et al (2013) Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-κB pathways. J Nutr Biochem 24:2040–2050
Ghoneum M, Abdulmalek S, Pan D (2020) Reversal of age-associated oxidative stress in mice by PFT, a novel kefir product. Int J Immunopathol Pharmacol 34:2058738420950149
Rikans LE, Hornbrook KR (1997) Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta 1362:116–127
Aoyama K, Nakaki T (2012) Inhibition of GTRAP3-18 may increase neuroprotective glutathione (GSH) synthesis. Int J Mol Sci 13:12017–12035
Wang Y et al (2018) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217:1915–1928
Furukawa S et al (2017) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761
Abo-Haded HM et al (2017) Hepatoprotective effect of sitagliptin against methotrexate induced liver toxicity. PLOS ONE 12:e0174295
Rahman I, MacNee W (2000) Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 16:534–554
Yamauchi A et al (2020) Placental extract ameliorates liver fibrosis in a methionine- and choline-deficient diet-induced mouse model of non-alcoholic steatohepatitis. Biomed Res 41:1–12
Cataldi M, Citro V, Resnati C, Manco F, Tarantino G (2021) New avenues for treatment and prevention of drug-induced steatosis and steatohepatitis: much more than antioxidants. Adv Ther 38:2094–2113
Funding
No funding was provided for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
Approval from the institutional ethics committee was obtained. Animal experiments were done with strict accordance to the institutional ethical guidelines for the care and use of animals in research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghoneum, M., El-Gerbed, M.S.A. Human placental extract ameliorates methotrexate-induced hepatotoxicity in rats via regulating antioxidative and anti-inflammatory responses. Cancer Chemother Pharmacol 88, 961–971 (2021). https://doi.org/10.1007/s00280-021-04349-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04349-4