Abstract
Purpose
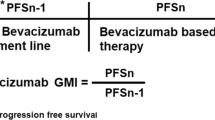
The aim of this study was to investigate the association between changes in the levels of vascular endothelial growth factors (VEGFs) after treatment with bevacizumab and gemcitabine (Bev-Gem) and the clinical outcome.
Methods
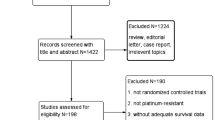
Platinum-resistant ovarian cancer patients treated with Bev-Gem therapy at our hospital between 2014 and 2018 were identified. Serum VEGF levels at the first and second treatment cycle were measured by ELISA. All patients were categorized into two groups—patients with > 50% decrease in serum VEGF-A levels (Group A) and patients with < 50% decrease serum VEGF-A levels (Group B). The association between clinical outcome and serum VEGF levels was investigated between the two groups.
Results
Among 18 patients, 10 were in Group A and 8 in Group B. Group A exhibited a lower response rate (0% vs.75% p < 0.01) and clinical benefit rate (60% vs.100% p = 0.02) than Group B. The median serum VEGF-A level of Group A before the first cycle of Bev-Gem therapy was higher than that in Group B (61.2 vs. 3.7 pg/mL, p < 0.01). Group A exhibited worse PFS (7 vs., 10 months, p < 0.01) and OS (17 vs. 26 months, p = 0.04) than Group B. There were more patients with > 10% increase in serum VEGF-B levels in Group A than in Group B (p < 0.01).
Conclusion
The rapid decrease in VEGF-A levels and the resultant increase in serum VEGF-B levels might be associated with an unfavorable clinical outcome. Large-scale studies are needed to further examine these results.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Lheureux S, Gourley C, Vergote I, Oza AM (2019) Epithelial ovarian cancer. Lancet 393:1240–1253. https://doi.org/10.1016/S0140-6736(18)32552-2
Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D, Ray-Coquard I (2014) Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol 32:1302–1308. https://doi.org/10.1200/JCO.2013.51.4489
Poveda AM, Selle F, Hilpert F, Reuss A, Savarese A, Vergote I, Witteveen P, Bamias A, Scotto N, Mitchell L, Pujade-Lauraine E (2015) Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in Platinum-Resistant Recurrent Ovarian Cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol 33:3836–3838. https://doi.org/10.1200/JCO.2015.63.1408
Takasaki K, Miyamoto M, Takano M, Soyama H, Aoyama T, Matsuura H, Kato K, Sakamoto T, Kuwahara M, Iwahashi H, Ishibashi H, Yoshikawa T, Furuya K (2018) Addition of bevacizumab to gemcitabine for platinum-resistant recurrent ovarian cancer: a retrospective analysis. Cancer Chemother Pharmacol 81:809–814. https://doi.org/10.1007/s00280-018-3552-5
Ferrara N, Hillan KJ, Novotny W (2005) Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 333:328–335. https://doi.org/10.1016/j.bbrc.2005.05.132
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3:391–400. https://doi.org/10.1038/nrd1381
Yang X, Zhang Y, Hosaka K, Andersson P, Wang J, Tholander F, Cao Z, Morikawa H, Tegnér J, Yang Y, Iwamoto H, Lim S, Cao Y (2015) VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc Natl Acad Sci USA 112:E2900–E2909. https://doi.org/10.1073/pnas.1503500112
Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Umemoto M, Sakamoto T, Sato S, Mizunuma H, Smith SK (2003) Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer 88:237–244. https://doi.org/10.1038/sj.bjc.6600701
Smerdel MP, Steffensen KD, Waldstrøm M, Brandslund I, Jakobsen A (2010) The predictive value of serum VEGF in multiresistant ovarian cancer patients treated with bevacizumab. Gynecol Oncol 118:167–171. https://doi.org/10.1016/j.ygyno.2010.03.018
Madsen CV, Steffensen KD, Olsen DA, Waldstrøm M, Smerdel M, Adimi P, Brandslund I, Jakobsen A (2012) Serial measurements of serum PDGF-AA, PDGF-BB, FGF2, and VEGF in multiresistant ovarian cancer patients treated with bevacizumab. J Ovarian Res 5:23. https://doi.org/10.1186/1757-2215-5-23
D'Agostino G, Amant F, Berteloot P, Scambia G, Vergote I (2003) Phase II study of gemcitabine in recurrent platinum-and paclitaxel-resistant ovarian cancer. Gynecol Oncol 88(3):266–269
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Mutch DG, Prat J (2014) FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol 133:401–404. https://doi.org/10.1016/j.ygyno.2014.04.013
Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A (2010) Ephrin-B2 regulates VEGFR2 function in developmental and tumor angiogenesis. Nature 465:487–491. https://doi.org/10.1038/nature08995
Cao Y (2009) Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal 2:re1. https://doi.org/10.1126/scisignal.259re1
Shibuya M (2006) Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 9:225–230. https://doi.org/10.1007/s10456-006-9055-8
Milross CG, Mason KA, Hunter NR, Chung WK, Peters LJ, Milas L (1996) Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst 88:1308–1314. https://doi.org/10.1093/jnci/88.18.1308
Lau DH, Xue L, Young LJ, Burke PA, Cheung AT (1999) Paclitaxel (Taxol): an inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother Radiopharm 14:31–36. https://doi.org/10.1089/cbr.1999.14.31
Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK (1999) Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res 59:3776–3782
Lopes NM, Adams EG, Pitts TW, Bhuyan BK (1993) Cell kill kinetics and cell cycle effects of taxol on human and hamster ovarian cell lines. Cancer Chemother Pharmacol 32:235–242. https://doi.org/10.1007/bf00685842
Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, Aoki D, Jobo T, Kodama S, Terauchi F, Sugiyama T, Ochiai K (2013) Japanese Gynecologic Oncology Group. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14:1020–1026. https://doi.org/10.1016/S1470-2045(13)70363-2
Bell C, Lynam E, Landfair DJ, Janjic N, Wiles ME (1999) Oligonucleotide NX1838 inhibits VEGF165-mediated cellular responses in vitro. Vitro Cell Dev Biol Anim 35:533–542. https://doi.org/10.1007/s11626-999-0064-y
Jain RK (2009) A new target for tumor therapy. N Engl J Med 360:2669–2671. https://doi.org/10.1056/NEJMcibr0902054
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Since our study was a retrospective analysis, formal informed consent was not obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soyama, H., Miyamoto, M., Matsuura, H. et al. Rapid decrease in serum VEGF-A levels may be a worse prognostic biomarker for patients with platinum-resistant recurrent ovarian cancer treated with bevacizumab and gemcitabine. Cancer Chemother Pharmacol 85, 941–947 (2020). https://doi.org/10.1007/s00280-020-04070-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04070-8