Abstract
Purpose
This open-label, multi-center phase II study investigated the efficacy and safety of the combination of 5-fluorouracil (5-FU)/folinic acid (FA) plus gemcitabine (GFF) in patients with advanced pancreatic cancer. The study is based on our completed dose finding phase I trial.
Methods
A total of 90 patients (pts) were recruited between 02/2000 and 04/2002 to receive 5-FU 750 mg/m2 (24 h, i.v.), FA 500 mg/m2 (2 h, i.v.) and gemcitabine 1,000 mg/m2 (30 min, i.v.) on days 1, 8, 15, and 22. Treatment was repeated on day 43 until disease progression. The primary objective was the 1-year survival rate. The trial was conducted in compliance with the Declaration of Helsinki.
Results
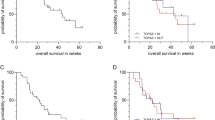
The 1-year survival rate was 25% [95% CI: 16–34], median overall survival was 6.8 months [95% CI: 5.13–8.45], 9 patients showed partial responses (PR) so that the overall response rate was 10.3%. Overall control rate (PR + stable disease for at least 6 months) was 56%. Median time to progression was 4.6 months [95% CI: 3.68–5.52]. In 402 GFF cycles, we observed adverse events grade 3 in up to 10% of patients and grade 4 below 5% of patients.
Conclusions
The GFF combination appears to be effective and well tolerated. This intravenous regimen represents an intensified therapy with low frequency of toxicities and seems to be convenient for patients who are unable to get oral anti-neoplastic medication. After these encouraging results, the German CONKO-002 trial investigated the GFF regimen versus single-agent gemcitabine.

Similar content being viewed by others
References
Stathis A, Moore MJ (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 7(3):163–172
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249
Bozzetti F (2009) Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer 17(3):279–284
Pelzer U, Arnold D, Gövercin M, Stieler J, Doerken B, Riess H, Oettle H (2010) Parenteral nutrition support for patients with pancreatic cancer. Results of a phase II study. BMC Cancer 10:86
Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C (2008) Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 8:82
Heinemann V, Philip PA, Pelzer U (2009) Accomplishments in 2008 in the treatment of metastatic pancreatic cancer. Gastrointest Cancer Res 3(5 Suppl 2):43–47
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966
Pelzer U, Kubica K, Stieler J, Schwaner I, Heil G, Görner M, Mölle M, Hilbig A, Dörken B, Riess H, Oettle H (2008) A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol 26
Lutz MP, Königer M, Muche R, Ellenrieder V, Steinkamp M, Adler G, Gress TM (1999) A phase II study of weekly 24-h infusion of high-dose 5-fluorouracil in advanced pancreatic cancer. Z Gastroenterol 37(10):993–997
Oettle H, Riess H (2002) Gemcitabine in combination with 5-fluorouracil with or without folinic acid in the treatment of pancreatic cancer. Cancer 95(4 Suppl):912–922
Oettle H, Pelzer U, Hochmuth K, Diebold T, Langrehr J, Schmidt CA, Arning M, Vogl TJ, Neuhaus P, Huhn D, Riess H (1999) Phase I trial of gemcitabine (Gemzar), 24 h infusion 5-fluorouracil and folinic acid in patients with inoperable pancreatic cancer. Anticancer Drugs 10(8):699–704
Van Rijswijk REN, Jeziorski K, Wagener DJT, Van Laethem J, Reuse S, Baron B, Wils J (2004) Weekly high-dose 5-fluorouracil and folinic acid in metastatic pancreatic carcinoma: a phase II study of the EORTC GastroIntestinal Tract Cancer Cooperative Group. Eur J Cancer 40(14):2077–2081
Roehrig S, Wein A, Albrecht H, Konturek PC, Reulbach U, Männlein G, Wolff K, Ostermeier N, Hohenberger W, Hahn EG, Boxberger F (2010) Palliative first-line treatment with weekly high-dose 5-fluorouracil as 24 h-infusion and gemcitabine in metastatic pancreatic cancer (UICC IV). Med Sci Monit 6(3):CR124–CR131
Shiah H, Cheng A, Hsu C, Hsu C, Liu T, Chang J, Jan C, Chao Y, Yu W, Chuang T, Whang-Peng J, Chen L (2006) Phase I-II trial of weekly gemcitabine plus high-dose 5-fluorouracil and leucovorin in advanced pancreatic cancer. J Gastroenterol Hepatol 21(3):531–536
Santasusana JM, García López JL, García JJ, Carbonero AIL, Plazas JG, Rovira PS, Martos CF, Guzmán MCG, Jericó JF, Delgado FJD, Espinosa JC, Muñoz ML, Aguilar EA, Valera JS, García Ribas I, Mena AC (2005) A phase II trial of gemcitabine and weekly high-dose 5-fluorouracil in a 48-h continuous-infusion schedule in patients with advanced pancreatic carcinoma. A study of the Spanish Cooperative Group for Gastrointestinal Tumour Therapy (TTD). Clin Transl Oncol 7(11):493–498
Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20(15):3270–3275
Di Costanzo F, Carlini P, Doni L, Massidda B, Mattioli R, Iop A, Barletta E, Moscetti L, Recchia F, Tralongo P, Gasperoni S (2005) Gemcitabine with or without continuous infusion 5-FU in advanced pancreatic cancer: a randomised phase II trial of the Italian oncology group for clinical research (GOIRC). Br J Cancer 93(2):185–189
Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, Falk S, Crellin A, Adab F, Thompson J, Leonard P, Ostrowski J, Eatock M, Scheithauer W, Herrmann R, Neoptolemos JP (2009) Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 27(33):5513–5518
Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B, Köhne C, Mingrone W, Stemmer SM, Tàmas K, Kornek GV, Koeberle D, Cina S, Bernhard J, Dietrich D, Scheithauer W (2007) Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 25(16):2212–2217
Riess H, Helm A, Niedergethmann M, Schmidt-Wolf I, Moik M, Hammer C, Zippel K, Weigang-Köhler K, Stauch M, Oettle H, Deutsche Krebsgesellschaft (2005) A randomised, prospective, multicenter, phase III Trial of Gemcitabine, 5-Fluorouracil (5-FU), Folinic Acid vs. Gemcitabine alone in Patients with Advanced Pancreatic Cancer. J Clin Oncol, ASCO Annual Meeting Proceedings, vol 23, No. 16S, Part I of II (Jun 1 Suppl) A 4009
Acknowledgments
This study was supported by “Deutsche Krebsgesellschaft e.V.”, the German AIO-Group and by a grand from Lilly Deutschland GmbH to the CONKO-study group.
Conflict of interest
The authors declare that they have no competing interest or financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pelzer, U., Arnold, D., Reitzig, P. et al. First-line treatment of pancreatic cancer patients with the combination of 5-fluorouracil/folinic acid plus gemcitabine: a multicenter phase II trial by the CONKO-study group. Cancer Chemother Pharmacol 68, 1173–1178 (2011). https://doi.org/10.1007/s00280-011-1602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1602-3