Abstract
Aim
A multi-centred, open-labelled, phase II study containing 46 patients was conducted to evaluate the clinical benefit of gemcitabine (1,400 mg/m2) combined with 5-FU (3 g/m2) in a 48 h continuous infusion (CI).
Methods
Both drugs were administered on days 1,8 and 15 of every 4 week cycle in chemotherapy-naïve patients with locally advanced un-resectable metastatic pancreatic carcinoma. The minimum follow-up was 6 months.
Results
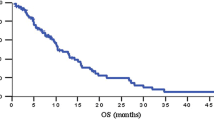
Clinical benefit response was the primary endopint and this was achieved by 24.4% of the patients. Quality of life (QoL) improved in 16.6% of patients. Objective response was observed in 7% of the patients. The median progression-free survival (PFS) was 14.4 weeks and the median overall survival (OS) time was 22.7 weeks. One-year survival was 25%. The most frequent grade 3–4 toxicities were neutropenia (4.5%), mucositis (7.5%) and hyperbilirubinaemia (10.5%).
Conclusions
This schedule was not superior in terms of clinical benefit, response rate, PFS and OS than standard gemcitabine treatment.
Similar content being viewed by others
References
Jensen OM, Esteve J, Moller H, et al. Cancer in the European Community and its member states. Eur J Cancer. 1990;26:1167–256.
Rothemberg ML, Moore MJ, Cripps MC, et al. A phase II trial of gemcitabine in patients with 5FU-refractory pancreas cancer. Ann Oncol. 1996;7:347–53.
Burris I HA, Moore MJ, Andersen J, et al. Improvement in survival and clinical benefit with gemcitabine as first line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–13.
Aranda A, Díaz-Rubio E, Cervantes A, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with weekly high-dose 48 hour continuous-infusion fluorouracil for advanced colorectal cancer: A Spanish Cooperative Group for Gastrointestinal Tumour Therapy (TTD) study. Ann Oncol. 1998;9:727–31.
Rodríguez-Lescure A, Carrato A, Massuti B, et al. Phase I/II study of gemcitabine and weekly 48-hour continuous infusion of high dose 5-fluorouracil in advanced exocrine pancreatic cancer. Proc Am Soc Clin Oncol. 1999;18:298.
Cascinu S, Silva RR, Barni S, et al. A combination of gemcitabine and 5-fluorourcacil in advanced pancreatic cancer, a report from the Italian Group for the Study of Digestive Tract Cancer (GISCAD). Br J Cancer. 1999; 80:1595–8.
Hidalgo M, Castellano D, Paz-Ares L, et al. Phase I–II study of gemcitabine and fluorouracil as a continuous infusion in patients with pancreatic cancer. J Clin Oncol. 1999;17:585–92.
Oettle H, Arning M, Pelzer U, et al. A phase II trial of gemcitabine in combination with 5-fluorouracil (24-hour) and folinic acid in patients with chemonaive advanced pancreatic cancer. Ann Oncol. 2000;11:1267–72.
Kurtz JE, Kohser F, Negrier S, et al. Gemcitabine and protracted 5FU for advanced pancreatic cancer: A phase II study. Hepatogastroenterology. 2000;47:1450–3.
Feliu J, López Álvarez MP, Jaraiz MA, et al. Phase II trial of gemcitabine and UFT by leucovorin in patients with advanced pancreatic carcinoma. The ONCOPAZ Cooperative Group. Cancer. 2000;89:1706–13.
Cascinu S, Frontini L, Labianca R, et al. A combination of a fixed dose rate infusion of gemcitabine associated to a bolus 5-fluorouracil in advanced pancreatic cancer, a report from the Italian Group for the Study of Digestive Tract Cancer (GISCAD) Ann Oncol. 2000;11:1309–11.
Berlin JD, Adak S, Vaughn DJ, et al. A phase II study of gemcitabine and 5-fluorouracil in metastatic pancreatic cancer: An Eastern Cooperative Oncology Group Study (E3296). Oncology. 2000;58:215–8.
Louvet C, Andre T, Hammel P, et al. Phase II trial of bimonthly leucovorin, 5-fluorouracil and gemcitabine for advanced pancreatic adenocarcinoma (FOLFUGEM). Ann Oncol. 2001;12:675–9.
Marantz A, Jovits, Almira E, et al. Phase II study of gemcitabine, 5-fluorouracil, and leucovorin in patients with pancreatic cancer. Semin Oncol. 2001;28:44–9.
Berlin J, Catalano P, Thomas J, et al. A phase III study of gemcitabine in combination with 5-FU versus gemcitabine alone in patients with advanced pancreatic adenocarcinoma (E2297): an Eastern Cooperative Oncology Group (ECOG) trial. Proc Am Soc Clin Oncol. 2001;20:127.
DiConstanzo F, Sdrobolini A, Carlini A, et al. Gemcitabine (GEM) alone or in combination with 5-FU continuous infusion (CI) in the treatment of advanced pancreatic cancer (APC): A GOIRC randomized phase II trial. Proc Am Soc Clin Oncol. 2001;20:612.
Scheithauer W, Schüll B, Ulrich-Pur H, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol. 2003:14:197–04.
Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First Report of Intergroup Trial C974·1/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santasusana, J.M., García López, J.L., Bretón García, J.J. et al. A phase II trial of gemcitabine and weekly high-dose 5-fluorouracil in a 48-hour continuous-infusion schedule in patients with advanced pancreatic carcinoma. A study of the Spanish Cooperative Group for Gastrointestinal Tumour Therapy (TTD). Clin Transl Oncol 7, 493–498 (2005). https://doi.org/10.1007/BF02717002
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02717002