Abstract
Purpose
This study was conducted to evaluate the safety and efficacy of S-1 and paclitaxel combination therapy for patients with advanced gastric cancer.
Methods
Eligible patients had previously untreated advanced or relapsed gastric cancer with measurable lesion(s) and an ECOG PS of 0-2. Treatment consisted of S-1 35 mg/m2 p.o. b.i.d. on days 1–14 followed by a 7-day off plus paclitaxel 70 mg/m2 i.v. on days 1 and 8 of a 21-day cycle.
Results
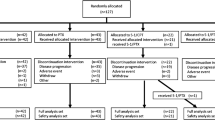
Fifty-six patients (M/F = 37/19) were enrolled. The median age was 59 years. The median number of cycles administered was six (range 1–18). Out of the 53 patients evaluated, there was 1 (1.9%) CR, 20 (37.7%) confirmed PRs, 5 (9.4%) unconfirmed PRs, 21 (39.6%) SDs, and 6 (11.3%) PDs. The objective tumor response was 39.6%. The median time to progression was 29 weeks. The median survival was 51 weeks. All 56 patients were assessed for treatment safety. The treatment was well tolerated with grade 3/4 neutropenia in 20%/13%, grade 3 febrile neutropenia in 7%, grade 2/3 diarrhea in 9%/4%, vomiting in 11%/0%, stomatitis in 4%/4%, and neuropathy in 4%/0% of patients.
Conclusions
S-1 and paclitaxel combination treatment is an effective regimen with a favorable toxicity profile in patients with advanced gastric cancer.



Similar content being viewed by others
References
Ajani JA, Ilson DH, Kelsen DP (1996) Paclitaxel in the treatment of patients with upper gastrointestinal carcinomas. Semin Oncol 23:55–58
Ajani JA, Lee FC, Singh DA, Haller DG, Lenz HJ, Benson AB 3rd, Yanagihara R, Phan AT, Yao JC, Strumberg D (2006) Multicenter phase II trial of S-1 plus cisplatin in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 24:663–667
Chollet P, Schoffski P, Weigang-Kohler K, Schellens JH, Cure H, Pavlidis N, Grunwald V, De Boer R, Wanders J, Fumoleau P (2003) Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer 39:1264–1270
Chu QS, Hammond LA, Schwartz G, Ochoa L, Rha SY, Denis L, Molpus K, Roedig B, Letrent SP, Damle B, DeCillis AP, Rowinsky EK (2004) Phase I and pharmacokinetic study of the oral fluoropyrimidine S-1 on a once-daily-for-28-day schedule in patients with advanced malignancies. Clin Cancer Res 10:4913–4921
D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 240:808–816
Fujitani K, Narahara H, Takiuchi H, Tsujinaka T, Satomi E, Gotoh M, Hirao M, Furukawa H, Taguchi T (2005) Phase I and pharmacokinetic study of S-1 combined with weekly paclitaxel in patients with advanced gastric cancer. Oncology 69:414–420
Fujiwara Y, Fujita J, Kan K, Tsukahara K, Takiguchi S, Miyata H, Yasuda T, Doki Y, Monden M (2006) A phase I study of combination chemotherapy using TS-1 and weekly paclitaxel for advanced gastric cancer. Gan To Kagaku Ryoho 33:45–48
Hoff PM, Saad ED, Ajani JA, Lassere Y, Wenske C, Medgyesy D, Dwivedy S, Russo M, Pazdur R (2003) Phase I study with pharmacokinetics of S-1 on an oral daily schedule for 28 days in patients with solid tumors. Clin Cancer Res 9:134–142
Hokita S, Aikou T, Miyazono F, Ishigami S, Aridome K, Maenohara S, Saihara T, Suenaga K, Nomura H, Maeda S, Takatori H, Arima H, Uchikado Y, Natsugoe S, Takao S (2006) A phase I combination chemotherapy study of biweekly paclitaxel and S-1 administration in patients with advanced gastric cancer. Cancer Chemother Pharmacol 57:736–740
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ (2004) Cancer statistics, 2004. CA Cancer J Clin 54:8–29
Jeung HC, Rha SY, Kim HK, Lim HY, Kim S, Kim SY, Gong SJ, Park CH, Ahn JB, Noh SH, Chung HC (2007) Multi-institutional phase II study of S-1 monotherapy in advanced gastric cancer with pharmacokinetic and pharmacogenomic evaluations. Oncologist 12:543–554
Kano Y, Akutsu M, Tsunoda S, Ando J, Matsui J, Suzuki K, Ikeda T, Inoue Y, Adachi K (1996) Schedule-dependent interaction between paclitaxel and 5-fluorouracil in human carcinoma cell lines in vitro. Br J Cancer 74:704–710
Kawabata R, Fujiwara Y, Doki Y, Fujita J, Tsukahara Y, Yamasaki M, Miyata H, Takiguchi S, Monden M (2007) Phase I/II study of a combination of S-1 and weekly paclitaxel in patients with advanced or recurrent gastric cancer. Oncology 72:219–225
Koizumi W, Kurihara M, Nakano S, Hasegawa K (2000) Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 58:191–197
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Gotro Korea (2002) Annual report on the cause of death statistics, 2001. Korea National Statistical Office, Daejeon
Mochiki E, Ohno T, Kamiyama Y, Aihara R, Haga N, Ojima H, Nakamura J, Ohsawa H, Nakabayashi T, Takeuchi K, Asao T, Kuwano H (2006) Phase I/II study of S-1 combined with paclitaxel in patients with unresectable and/or recurrent advanced gastric cancer. Br J Cancer 95:1642–1647
Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO (1999) Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol 22:580–586
N. Boku SY, Shirao, K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Kimura A, Ohtsu A, Gastrointestinal Oncology Study Group/Japan Clinical Oncology Group (2007) Randomized phase III study of 5-fluorouracil (5-FU) alone versus combination of irinotecan and cisplatin (CP) versus S-1 alone in advanced gastric cancer (JCOG9912). J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 25;18S:LBA4513
Nakajo A, Hokita S, Ishigami S, Miyazono F, Etoh T, Hamanoue M, Maenohara S, Iwashita T, Komatsu H, Satoh K, Aridome K, Morita S, Natsugoe S, Takiuchi H, Nakano S, Maehara Y, Sakamoto J, Aikou T (2008) A multicenter phase II study of biweekly paclitaxel and S-1 combination chemotherapy for unresectable or recurrent gastric cancer. Cancer Chemother Pharmacol
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Sugimachi K, Maehara Y, Horikoshi N, Shimada Y, Sakata Y, Mitachi Y, Taguchi T (1999) An early phase II study of oral S-1, a newly developed 5-fluorouracil derivative for advanced and recurrent gastrointestinal cancers. The S-1 Gastrointestinal Cancer Study Group. Oncology 57:202–210
Ueda Y, Yamagishi H, Ichikawa D, Morii J, Koizumi K, Kakihara N, Shimotsuma M, Takenaka A, Yamashita T, Kurioka H, Nishiyama M, Morita S, Nakamura K, Sakamoto J (2005) Phase I study of a combination of s-1 and weekly paclitaxel in patients with advanced or recurrent gastric cancer. Oncology 69:261–268
Wils J (1996) The treatment of advanced gastric cancer. Semin Oncol 23:397–406
Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, Sato Y, Todo S, Terashima M, Gotoh M, Sakamoto J, Nishiyama M (2006) Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 12:3402–3407
Acknowledgments
This study was partially supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (0412-CR01-0704-0001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.J., Kim, SY., Chung, HC. et al. A multi-center phase II study of S-1 plus paclitaxel as first-line therapy for patients with advanced or recurrent unresectable gastric cancer. Cancer Chemother Pharmacol 63, 1083–1090 (2009). https://doi.org/10.1007/s00280-008-0818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0818-3