Abstract
Background
The aim of this study was to explore whether a combination of S-1 and paclitaxel offers any benefit over paclitaxel alone to patients pretreated by S-1.
Methods
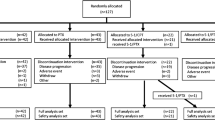
Gastric cancer patients who developed progression during S-1-based first-line chemotherapy or had recurrence during postoperative adjuvant chemotherapy by S-1 were randomly assigned to receive second-line treatment either by weekly administration of paclitaxel at 80 mg/m2 three times every 4 weeks or daily oral S-1 (80 mg/m2) for 2 weeks plus paclitaxel (50 mg/m2) given on days 1 and 8, every 3 weeks (S-1 plus paclitaxel). The primary endpoint was progression-free survival (PFS) at 4 months after the initiation of treatment.
Results
A total of 78 patients were eligible for efficacy analyses—40 were assigned to the paclitaxel group and 38 to the S-1 plus paclitaxel group. PFS at 4 months was similar between the groups (50 % for paclitaxel vs 55 % for S-1 plus paclitaxel, P = 0.641). There were no differences between the groups either in progression-free survival (4.6 vs 4.6 months, respectively, P = 0.526), overall survival (10.0 vs 10.0 months, respectively, P = 0.464), or overall response rate (27 vs 22 %, respectively, P = 0.767). The incidences of grade 3 or 4 hematological and non-hematological toxicities were also equivalent between the two groups (25 vs 26 % and 24 vs 26 %, respectively).
Conclusions
No benefit of S-1 administration beyond progression was shown when paclitaxel was selected as the key drug for second-line chemotherapy.




Similar content being viewed by others
References
GLOBOCAN (2012) http://globocan.iarc.fr. Accessed 1 Aug 2015
Shirasaka T, Shimamoto Y, Ohshimo H et al (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drug 7:548–557
Thuss-Patience PC, Kretzschmar A, Bichev D et al (2011) Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer: a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 47:2306–2314
Kang JH, Lee SI, Lim DH et al (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30:1513–1518
Hironaka S, Ueda S, Yasui H et al (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 31:4438–4444
Nukatsuka M, Fujioka A, Nakagawa F et al (2004) Antimetastatic and anticancer activity of S-1, a new oral dihydropyrimidine -dehydrogenase-inhibiting fluoropyrimidine, alone and in combination with paclitaxel in an orthotopically implanted human breast cancer model. Int J Oncol 25:1531–1536
Sakurai Y, Yoshida I, Kamoshida S et al (2008) Effects of combined administration of DPD-inhibitory oral fluoropyrimidine, S-1, plus paclitaxel on gene expressions of fluoropyrimidine metabolism-related enzymes in human gastric xenografts. Ann Surg Oncol 15:2301–2309
Uedo N, Narahara H, Ishihara R et al (2007) Phase II study of a combination of irinotecan and S-1 in patients with advanced gastric cancer (OGSG0002). Oncology 73:65–71
Narahara H, Fujitani K, Takiuchi H et al (2008) Phase II study of a combination of S-1 and paclitaxel in patients with unresectable or metastatic gastric cancer. Oncology 74:37–41
Yoshida K, Ninomiya M, Takakura N et al (2006) Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 12:3402–3407
Rothenberg ML, Oza AM, Bigelow RH et al (2003) Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol 21:2059–2069
Tournigand C, André T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Kodera Y, Ito S, Mochizuki Y et al (2007) A phase II study of weekly paclitaxel as second-line chemotherapy for advanced gastric cancer (CCOG0302 study). Anticancer Res 27:2667–2671
Koizumi W, Tanabe S, Saigenji K et al (2003) Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer 89:2207–2212
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Koizumi W, Kim YH, Fujii M, JACCRO and KCSG Study Group et al (2014) Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 140:319–328
Narahara H, Iishi H, Imamura H et al (2011) Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer 14:72–80
Mochizuki Y, Ohashi N, Kojima H et al (2013) CPT-11 as a second-line treatment for patients with advanced/metastatic gastric cancer who failed S-1 (CCOG0702). Cancer Chemother Pharmacol 72:629–635
Nishikawa K, Tanabe K, Fujii M et al (2014) A randomized phase III trial of second-line chemotherapy comparing CPT-11 alone versus S-1 plus CPT-11 combination therapy in advanced gastric cancer refractory to first-line therapy with S-1 (JACCRO GC-05). J Clin Oncol 32(suppl 3), abstr 87
Shitara K, Muro K, Ura T et al (2008) Chemotherapy for gastric cancer that recurs after adjuvant chemotherapy with S-1. Jpn J Clin Oncol 38:786–789
Takahashi M, Tsuburaya A, Nishikawa K et al (2015) A phase II trial of capecitabine plus cisplatin (XP) for patients with advanced gastric cancer who relapsed after S-1 adjuvant therapy, XP after TS-1 adjuvant failure (XParTS). J Clin Oncol 33(suppl 3), (abstr 124)
Kim J, Sun CL, Mailey B et al (2010) Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol 21:152–160
Fuchs CS, Tomasek J, Yong CJ, REGARD Trial Investigators et al (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31–33
Wilke H, Muro K, Van Cutsem E, RAINBOW Study Group et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235
Acknowledgments
We thank the non-profit organization Epidemiological and Clinical Research Information Network (ECRIN) for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Morita has received grants and personal fees from Taiho Pharmaceutical Company, grants and personal fees from Bristol Myers Squib, outside the submitted work. Kodera has received grants and personal fees from Taiho Pharmaceutical Company, grants and personal fees from Bristol Meyers Squib, grants from Nippon Kayaku, outside the submitted work.
About this article
Cite this article
Nakanishi, K., Kobayashi, D., Mochizuki, Y. et al. Phase II multi-institutional prospective randomized trial comparing S-1 plus paclitaxel with paclitaxel alone as second-line chemotherapy in S-1 pretreated gastric cancer (CCOG0701). Int J Clin Oncol 21, 557–565 (2016). https://doi.org/10.1007/s10147-015-0919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0919-z