Abstract
Purpose
Second-line chemotherapy is now considered a standard therapy option in patients with advanced gastric cancer (AGC) who failed from first-line chemotherapy. Single agents, such as irinotecan, docetaxel or paclitaxel, provided an overall response rate of about 10 %. However, the efficacy was not satisfactory. The authors conducted a phase II study to investigate biweekly regimen of S-1 plus paclitaxel in Chinese AGC in second-line setting, with response rate as the primary end point.
Patients and methods
Patients with AGC failed from first-line chemotherapy with fluoropyrimidine/platinum who had measurable lesions were enrolled. Paclitaxel was administered intravenously on day 1 at a dose of 120 mg/m2, and oral S-1 was administered twice a day from days 1 to 7, followed by a 7-day drug-free interval.
Results
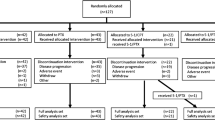
A total of 30 patients with pretreated AGC were accrued. No complete responses were observed. Partial responses were documented in 10 (33.3 %) patients. Ten (33.3 %) patients had stable disease. The median progression-free survival was 3.6 months and the overall survival was 7.2 months. The main toxicity was bone marrow suppression. The most frequent grade 3/4 hematological toxicities were neutropenia and anemia, which were observed in 8 (26.7 %) and 6 (20 %) patients, respectively. The most common grade 3/4 non-hematological toxicity was neuropathy, which was reported in 4 (13.3 %) patients.
Conclusion
Biweekly S-1 plus paclitaxel showed promising activity with acceptable toxicities as second-line chemotherapy in pretreated patients with AGC. This regimen deserves further investigation in a phase III trial.


Similar content being viewed by others
References
Zhu X, Li J (2010) Gastric carcinoma in China: current status and future perspectives (Review). Oncol Lett 1(3):407–412. doi:10.3892/ol_00000071
Cervantes A, Roda D, Tarazona N, Rosello S, Perez-Fidalgo JA (2013) Current questions for the treatment of advanced gastric cancer. Cancer Treat Rev 39(1):60–67. doi:10.1016/j.ctrv.2012.09.007
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24(31):4991–4997. doi:10.1200/JCO.2006.06.8429
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. doi:10.1016/S1470-2045(08)70035-4
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE (2010) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev (3):CD004064. doi:10.1002/14651858.CD004064.pub3
Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P (2011) Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 47(15):2306–2314. doi:10.1016/j.ejca.2011.06.002
Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY, Kang WK, Park SH (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30(13):1513–1518. doi:10.1200/JCO.2011.39.4585
Cook N, Marshall A, Blazeby J, Coxon F, Mansoor W, Bridgewater J (2013) Cougar-02: a randomized phase III study of docetaxel versus active symptom control in patients with relapsed esophago-gastric adenocarcinoma. J Clin Oncol 31(suppl):abstr 4023
Ueda S, Hironaka S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T (2012) Randomized phase III study of irinotecan (CPT-11) versus weekly paclitaxel (wPTX) for advanced gastric cancer (AGC) refractory to combination chemotherapy (CT) of fluoropyrimidine plus platinum (FP): WJOG4007 trial. J Clin Oncol 15. AMER SOC CLINICAL ONCOLOGY 2318 MILL ROAD, STE 800, ALEXANDRIA, VA 22314 USA
Nukatsuka M, Fujioka A, Nakagawa F, Oshimo H, Kitazato K, Uchida J, Sugimoto Y, Nagayama S, Fukushima M (2004) Antimetastatic and anticancer activity of S-1, a new oral dihydropyrimidine-dehydrogenase-inhibiting fluoropyrimidine, alone and in combination with paclitaxel in an orthotopically implanted human breast cancer model. Int J Oncol 25(6):1531–1536
Sakurai Y, Yoshida I, Kamoshida S, Inaba K, Isogaki J, Komori Y, Uyama I, Tsutsumi Y (2008) Effects of combined administration of DPD-inhibitory oral fluoropyrimidine, S-1, plus paclitaxel on gene expressions of fluoropyrimidine metabolism-related enzymes in human gastric xenografts. Ann Surg Oncol 15(8):2301–2309
Sakurai Y, Yoshida I, Kamoshida S, Inaba K, Isogaki J, Komori Y, Uyama I, Tsutsumi Y (2008) Effects of combined administration of DPD-inhibitory oral fluoropyrimidine, S-1, plus paclitaxel on gene expressions of fluoropyrimidine metabolism-related enzymes in human gastric xenografts. Ann Surg Oncol 15(8):2301–2309. doi:10.1245/s10434-008-9963-5
Mochiki E, Ohno T, Kamiyama Y, Aihara R, Haga N, Ojima H, Nakamura J, Ohsawa H, Nakabayashi T, Takeuchi K, Asao T, Kuwano H (2006) Phase I/II study of S-1 combined with paclitaxel in patients with unresectable and/or recurrent advanced gastric cancer. Br J Cancer 95(12):1642–1647. doi:10.1038/sj.bjc.6603497
Nakajo A, Hokita S, Ishigami S, Miyazono F, Etoh T, Hamanoue M, Maenohara S, Iwashita T, Komatsu H, Satoh K, Aridome K, Morita S, Natsugoe S, Takiuchi H, Nakano S, Maehara Y, Sakamoto J, Aikou T (2008) A multicenter phase II study of biweekly paclitaxel and S-1 combination chemotherapy for unresectable or recurrent gastric cancer. Cancer Chemother Pharmacol 62(6):1103–1109. doi:10.1007/s00280-008-0693-y
Ueda Y, Yamagishi H, Ichikawa D, Okamoto K, Otsuji E, Morii J, Koizumi K, Kakihara N, Shimotsuma M, Yamashita T, Taniguchi F, Aragane H, Nishi H, Itokawa Y, Morita S, Sakamoto J (2010) Multicenter phase II study of weekly paclitaxel plus S-1 combination chemotherapy in patients with advanced gastric cancer. Gastric Cancer 13(3):149–154. doi:10.1007/s10120-010-0548-1
Sugimoto N, Fujitani K, Imamura H, Uedo N, Iijima S, Imano M, Shimokawa T, Kurokawa Y, Furukawa H, Goto M (2014) Randomized phase II trial of S-1 plus irinotecan versus S-1 plus paclitaxel as first-line treatment for advanced gastric cancer (OGSG0402). Anticancer Res 34(2):851–857
Eisenhauer E, Therasse P, Bogaerts J, Schwartz L, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Hokita S, Aikou T, Miyazono F, Ishigami S, Aridome K, Maenohara S, Saihara T, Suenaga K, Nomura H, Maeda S, Takatori H, Arima H, Uchikado Y, Natsugoe S, Takao S (2006) A phase I combination chemotherapy study of biweekly paclitaxel and S-1 administration in patients with advanced gastric cancer. Cancer Chemother Pharmacol 57(6):736–740. doi:10.1007/s00280-005-0122-4
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10
Baek SK, Kim SY, Jeong JH, Cho KS, Yoon HJ (2012) Second-line chemotherapy for advanced gastric cancer in Korea. Gastric Cancer 15(4):345–354. doi:10.1007/s10120-011-0114-5
Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, Kim TY, Ryu MH, Nam BH, Zang DY (2013) Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol 24(11):2850–2854. doi:10.1093/annonc/mdt351
Takashima A, Boku N, Kato K, Nakamura K, Mizusawa J, Fukuda H, Shirao K, Shimada Y, Ohtsu A (2013) Survival prolongation after treatment failure of first-line chemotherapy in patients with advanced gastric cancer: combined analysis of the Japan Clinical Oncology Group Trials JCOG9205 and JCOG9912. Gastric Cancer. doi:10.1007/s10120-013-0309-z
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet 383(9911):31–39
Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H (2013) Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 31(26):3219–3225. doi:10.1200/JCO.2013.48.8585
Vasile E, Caparello C, Caponi S, Ginocchi L, Vivaldi C, Musettini G, Lucchesi M, Lencioni M, Falcone A (2014) Not only chemotherapy in the second-line treatment of metastatic gastric cancer. Ann Oncol 25(2):544–545. doi:10.1093/annonc/mdt570
Takiuchi H (2011) Second-line chemotherapy for gastric cancer: a new issue lies ahead in global trials. Gastric Cancer 14(3):206–211. doi:10.1007/s10120-011-0072-y
Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N, Takeda Y, Moriwaki T, Amagai K, Sekikawa T, Sakuyama T, Kanda T, Sasaki T, Azuma M, Takahashi F, Takeuchi M, Koizumi W (2014) Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer. doi:10.1016/j.ejca.2014.01.020
Baize N, Abakar-Mahamat A, Mounier N, Berthier F, Caroli-Bosc FX (2009) Phase II study of paclitaxel combined with capecitabine as second-line treatment for advanced gastric carcinoma after failure of cisplatin-based regimens. Cancer Chemother Pharmacol 64(3):549–555. doi:10.1007/s00280-008-0903-7
Zhang XT, Li J, Bai Y, Chu YP, Li J, Li Y, Gong JF, Shen L (2013) A phase II study of triweekly paclitaxel and capecitabine combination therapy in patients with fluoropyrimidine–platinum-resistant metastatic gastric adenocarcinoma. J Cancer Res Ther 9(Suppl):S153–S157. doi:10.4103/0973-1482.122512
Lamont EB, Schilsky RL (1999) The oral fluoropyrimidines in cancer chemotherapy. Clin Cancer Res 5(9):2289–2296
Nishikawa K, Tanabe K, Fujii M, Kunisaki C, Tsuji A, Matsuhashi N, Takagane A, Ohno T, Kawase T, Kochi M A (2014) Randomized phase III trial of second-line chemotherapy comparing CPT-11 alone versus S-1 plus CPT-11 combination therapy in advanced gastric cancer refractory to first-line therapy with S-1 (JACCRO GC-05). J Clin Oncol 3. AMER SOC CLINICAL ONCOLOGY 2318 MILL ROAD, STE 800, ALEXANDRIA, VA 22314 USA
Lee MJ, Hwang IG, Jang J-S, Choi JH, Park B-B, Chang MH, Kim ST, Park SH, Kang MH, Kang JH (2012) Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res Treat 44(4):235–241
Shim HJ, Yun JY, Hwang JE, Bae WK, Cho SH, Chung IJ (2011) Prognostic factor analysis of third-line chemotherapy in patients with advanced gastric cancer. Gastric Cancer 14(3):249–256
Acknowledgments
The all-important thanks should be given to the patients and family members participating in this study. This research was partly supported by Zhejiang Provincial Natural Science Fund (LY13H160007) and Zhejiang Medicines & Health Science and Technology Project (201348801).
Conflict of interest
The authors have declared no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, Y., Fang, W., Mao, C. et al. Biweekly S-1 plus paclitaxel (SPA) as second-line chemotherapy after failure from fluoropyrimidine and platinum in advanced gastric cancer: a phase II study. Cancer Chemother Pharmacol 74, 503–509 (2014). https://doi.org/10.1007/s00280-014-2537-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2537-2