Abstract
Purpose
The aims were to determine the maximum tolerable dose (MTD) of docetaxel with CYP3A inhibition by ketoconazole, and to correlate the pharmacokinetics of docetaxel with midazolam phenotyping of CYP3A activity.
Methods
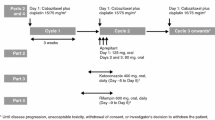
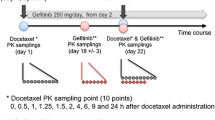
Forty-one patients with refractory metastatic cancers were treated with an escalating dose of intravenous docetaxel once in every 3 week of 10 mg/m2, concurrently with oral ketoconazole 200 mg twice daily for 3 days starting 2 days before the administration of docetaxel. Midazolam phenotyping test with ketoconazole modulation was performed before the first cycle of docetaxel. Docetaxel and midazolam pharmacokinetics were compared to our previous study of docetaxel treatment without ketoconazole modulation.
Results
Neutropenia was the dose-limiting toxicity. The maximum tolerated dose was 70 mg with mean AUC at 70 mg similar to 75 mg/m2 of docetaxel without ketoconazole. The plasma clearances of docetaxel and midazolam were reduced by 1.7- and 6-fold, respectively. The variability of midazolam AUC was reduced from 157 to 67%, but variability of docetaxel clearance was not reduced by CYP3A inhibition. Docetaxel clearance correlated with renal function and maximum concentration of ketoconazole, but not midazolam clearance or other variables of hepatic function.
Conclusion
Fixed dosing was found to be feasible, without increased variability of clearance or neutrophil toxicity compared to BSA-based dosing. With ketoconazole modulation, docetaxel clearance correlated with renal function but not CYP3A phenotype.


Similar content being viewed by others
References
Baker SD, van Schaik RH, Rivory LP, Ten Tije AJ, Dinh K, Graveland WJ, Schenk PW, Charles KA, Clarke SJ, Carducci MA, McGuire WP, Dawkins F, Gelderblom H, Verweij J, Sparreboom A (2004) Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res 10:8341–8350
Bourrie M, Meunier V, Berger Y, Fabre G (1996) Cytochrome P450 isoform inhibitors as a tool for the investigation of metabolic reactions catalyzed by human liver microsomes. J Pharmacol Exp Ther 277:321–332
Boxenbaum H (1999) Cytochrome P450 3A4 in vivo ketoconazole competitive inhibition: determination of Ki and dangers associated with high clearance drugs in general. J Pharm Pharm Sci 2:47–52
Boxenbaum H (1999) Human in vivo competitive inhibition of P450 substrates: increased plasma concentrations as a function of hepatic extraction ratio and percent inhibition. J Pharm Pharm Sci 2:89–91
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16:187–196
Bruno R, Vivier N, Veyrat-Follet C, Montay G, Rhodes GR (2001) Population pharmacokinetics and pharmacokinetic–pharmacodynamic relationships for docetaxel. Invest New Drugs 19:163–169
Chen YL, Felder L, Jiang X, Naidong W (2002) Determination of ketoconazole in human plasma by high-performance liquid chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 774:67–78
Clarke SJ, Rivory LP (1999) Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 36:99–114
D’Argenio DZ, Schumitzky A (1997) ADAPT II user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resources, Los Angeles
Engels FK, Loos WJ, Mathot RA, van Schaik RH, Verweij J (2007) Influence of ketoconazole on the fecal and urinary disposition of docetaxel. Cancer Chemother Pharmacol 60(4):569–579
Engels FK, Mathot RA, Loos WJ, van Schaik RH, Verweij J (2006) Influence of high-dose ketoconazole on the pharmacokinetics of docetaxel. Cancer Biol Ther 5:833–839
Engels FK, Ten Tije AJ, Baker SD, Lee CK, Loos WJ, Vulto AG, Verweij J, Sparreboom A (2004) Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther 75:448–454
Gibbs MA, Thummel KE, Shen DD, Kunze KL (1999) Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab Dispos 27:180–187
Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, Schuetz E, Lim R, Lim HL, Ong AB, Lee HS (2002) Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol 20:3683–3690
Gorski JC, Hall SD, Jones DR, VandenBranden M, Wrighton SA (1994) Regioselective biotransformation of midazolam by members of the human cytochrome P450 3A (CYP3A) subfamily. Biochem Pharmacol 47:1643–1653
Guitton J, Cohen S, Tranchand B, Vignal B, Droz JP, Guillaumont M, Manchon M, Freyer G (2005) Quantification of docetaxel and its main metabolites in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 19:2419–2426
Gurney H (1996) Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J Clin Oncol 14:2590–2611
Hirth J, Watkins PB, Strawderman M, Schott A, Bruno R, Baker LH (2000) The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res 6:1255–1258
Kharasch ED, Thummel KE, Watkins PB (2005) CYP3A probes can quantitatively predict the in vivo kinetics of other CYP3A substrates and can accurately assess CYP3A induction and inhibition. Mol Interv 5:151–153
Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R (1996) Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res 56:1296–1302
Martinez C, Garcia-Martin E, Pizarro RM, Garcia-Gamito FJ, Agundez JA (2002) Expression of paclitaxel-inactivating CYP3A activity in human colorectal cancer: implications for drug therapy. Br J Cancer 87:681–686
Maurice M, Pichard L, Daujat M, Fabre I, Joyeux H, Domergue J, Maurel P (1992) Effects of imidazole derivatives on cytochromes P450 from human hepatocytes in primary culture. FASEB J 6:752–758
Miyoshi Y, Ando A, Takamura Y, Taguchi T, Tamaki Y, Noguchi S (2002) Prediction of response to docetaxel by CYP3A4 mRNA expression in breast cancer tissues. Int J Cancer 97:129–132
Miyoshi Y, Taguchi T, Kim SJ, Tamaki Y, Noguchi S (2005) Prediction of response to docetaxel by immunohistochemical analysis of CYP3A4 expression in human breast cancers. Breast Cancer 12:11–15
Puisset F, Chatelut E, Dalenc F, Busi F, Cresteil T, Azema J, Poublanc M, Hennebelle I, Lafont T, Chevreau C, Roche H (2004) Dexamethasone as a probe for docetaxel clearance. Cancer Chemother Pharmacol 54:265–272
Reilly JJ, Workman P (1993) Normalisation of anti-cancer drug dosage using body weight and surface area: is it worthwhile? A review of theoretical and practical considerations. Cancer Chemother Pharmacol 32:411–418
Rosing H, Lustig V, van Warmerdam LJ, Huizing MT, ten Bokkel Huinink WW, Schellens JH, Rodenhuis S, Bult A, Beijnen JH (2000) Pharmacokinetics and metabolism of docetaxel administered as a 1-h intravenous infusion. Cancer Chemother Pharmacol 45:213–218
Sawyer M, Ratain MJ (2001) Body surface area as a determinant of pharmacokinetics and drug dosing. Invest New Drugs 19:171–177
Shou M, Martinet M, Korzekwa KR, Krausz KW, Gonzalez FJ, Gelboin HV (1998) Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics 8:391–401
Sparreboom A, Van Tellingen O, Scherrenburg EJ, Boesen JJ, Huizing MT, Nooijen WJ, Versluis C, Beijnen JH (1996) Isolation, purification, and biological activity of major docetaxel metabolites from human feces. Drug Metab Dispos 24:655–658
Toyo’oka T, Kumaki Y, Kanbori M, Kato M, Nakahara Y (2003) Determination of hypnotic benzodiazepines (alprazolam, estazolam, and midazolam) and their metabolites in rat hair and plasma by reversed-phase liquid-chromatography with electrospray ionization mass spectrometry. J Pharm Biomed Anal 30:1773–1787
Van Veldhuizen PJ, Reed G, Aggarwal A, Baranda J, Zulfiqar M, Williamson S (2003) Docetaxel and ketoconazole in advanced hormone-refractory prostate carcinoma: a phase I and pharmacokinetic study. Cancer 98:1855–1862
Yamamoto N, Tamura T, Kamiya Y, Sekine I, Kunitoh H, Saijo N (2000) Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J Clin Oncol 18:2301–2308
Yamamoto N, Tamura T, Murakami H, Shimoyama T, Nokihara H, Ueda Y, Sekine I, Kunitoh H, Ohe Y, Kodama T, Shimizu M, Nishio K, Ishizuka N, Saijo N (2005) Randomized pharmacokinetic and pharmacodynamic study of docetaxel: dosing based on body-surface area compared with individualized dosing based on cytochrome P450 activity estimated using a urinary metabolite of exogenous cortisol. J Clin Oncol 23:1061–1069
Yamaoka K, Nakagawa T, Uno T (1978) Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 6:165–175
Acknowledgments
We would like to thank NHG small innovative grant and MAP-RISE program for their support of the study.
Conflict of interest
All authors express no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, WP., Wang, LZ., Tham, LS. et al. A phase I study of docetaxel with ketoconazole modulation in patients with advanced cancers. Cancer Chemother Pharmacol 62, 243–251 (2008). https://doi.org/10.1007/s00280-007-0598-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0598-1