Abstract
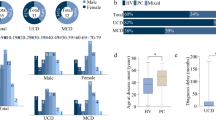
The purpose is to ascertain the clinical impact of Castleman disease (CD) by reassessment of the real-world data from Peking University First Hospital (PKUFH). The results will contribute to the standardization of diagnosis and treatment on CDs. Based on the last 15-year retrospective real-world data from Peking University First Hospital (PKUFH), we reclassified and re-evaluated the clinical and pathological information of patients with pathologically suspected diagnosis of CD. A total of 203 patients were included in our study, in which the diagnosis of CD was confirmed in 189 cases, including 118 patients with unicentric CD (UCD, n = 118, 62.4%) and 71 patients with multicentric CD (MCD, n = 71, 37.6%). A total of 44.1% (n = 52) of UCDs in our cohort were complicated with paraneoplastic pemphigus (PNP). The treatment of UCD is primarily surgical, with a 5-year overall survival (OS) of 88.1%. Patients with PNP had a poorer prognosis than those without PNP (82.9% (95% CI 123–178) vs 92.8% (95% CI 168–196), log-rank p = 0.041). The rate of concurrent systemic symptoms was 74.6% (n = 53), and renal involvement occurred in 49.3% (n = 35) MCD patients. The MCD treatments were mainly chemotherapy regimens, with a 5-year OS of 77.6% (95% CI, 143–213). Patients with UCD demonstrate a better overall prognosis than patients with MCD. But the prognosis of those complicated with PNP was poor. The differential diagnosis of MCD is extensive. MCD treatment in China is heterogeneous. The inaccessibility of anti-IL-6–targeted drugs in China may contribute to the poor prognosis for patients with MCD.
A preprint has previously been published (Guo et al. 34).



Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding authors.
References
Carbone A, Borok M, Damania B et al (2021) Castleman disease. Blood. 7(84):1–18
Munshi N, Mehra M, van de Velde H et al (2015) Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma 56:1252–1260
Lu Z, Li Z, Cao X et al (2016) Clinical spectrum and survival analysis of 145 cases of HIV-negative Castleman’s disease: renal function is an important prognostic factor[J]. Sci Rep 6:23831
Dong Y, Wang M, Nong L et al (2015) Clinical and laboratory characterization of 114 cases of Castleman disease patients from a single centre: paraneoplastic pemphigus is an unfavourable prognostic factor. Br J Haematol 169(6):834–842
Umehara H, Okazaki K, Kawa S et al (2021) Research program for intractable disease by the Ministry of Health, Labor and Welfare (MHLW) Japan. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol 31(3):529–533
Dispenzieri A, Fajgenbaum DC (2020) Overview of Castleman disease. Blood. 135(16):1353–1364
Jiang JP, Shen XF, Du JF et al (2018) A retrospective study of 34 patients with unicentric and multicentric Castleman’s disease: experience from a single institution. Oncol Lett 15(2):2407–2412
Fajgenbaum DC, Uldrick TS, Bagg A et al (2017) International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 129:1646–1657
van Rhee F, Voorhees P, Dispenzieri A et al (2018) International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 132(20):2115–2124
van Rhee F, Oksenhendler E, Srkalovic G et al (2020) International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv 4(23):6039–6050
Dong Y, Zhang L, Nong L et al (2018) Effectiveness of rituximab-containing treatment regimens in idiopathic multicentric Castleman disease. Ann Hematol 97(9):1641–1647
Dong Y, Na J, Lv J et al (2009) Clinical and laboratory characterization of a large cohort of patients with Castleman disease retrospectively collected from a single center. Leuk Lymphoma 50(8):1308–1317
Hematology Committee of Chinese Medical Association; Hematological Oncology Committee of China Anti-Cancer Association; China Castleman Disease Network (CCDN) (2021) The consensus of the diagnosis and treatment of Castleman disease in China (2021). Zhonghua Xue Ye Xue Za Zhi 42(7):529–534
Yoshito N, Fajgenbaum David C, Pierson Sheila K et al (2021) Validated international definition of the thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly clinical subtype (TAFRO) of idiopathic multicentric Castleman disease. Am J Hematol 96:1241–1252
Zhou T, Wang HW, Pittaluga S et al (2021) Multicentric Castleman disease and the evolution of the concept. Pathologica. 113(5):339–354
Facchetti F, Simbeni M, Lorenzi L (2021) Follicular dendritic cell sarcoma. Pathologica. 113(5):316–329
Shiroshita K, Kikuchi T, Okayama M et al (2020) Interleukin-6-producing intravascular large B-cell lymphoma with lymphadenopathy mimicking the histology of multicentric Castleman disease. Intern Med 59(23):3061–3065
Pina-Oviedo S, Miranda RN, Lin P et al (2017) Follicular lymphoma with hyaline-vascular Castleman-like features: analysis of 6 cases and review of the literature. Hum Pathol 68:136–146
Sato Y, Kojima M, Takata K et al (2010) Multicentric Castleman’s disease with abundant IgG4-positive cells: a clinical and pathological analysis of six cases. J Clin Pathol 63(12):1084–1089
Nishikori A, Nishimura MF, Nishimura Y et al (2021) Investigation of IgG4-positive cells in idiopathic multicentric Castleman disease and validation of the 2020 exclusion criteria for IgG4-related disease. Pathol Int 72(1):43–52
Otani K, Inoue D, Fujikura K et al (2018) Idiopathic multicentric Castleman’s disease: a clinicopathologic study in comparison with IgG4-related disease. Oncotarget. 9(6):6691–6706
Larroche C, Cacoub P, Soulier J et al (2002) Castleman’s disease and lymphoma: report of eight cases in HIV-negative patients and literature review. Am J Hematol 69(2):119–126
Wang L, Nong L, Li F et al (2020) Predominant stroma-rich feature in hyaline vascular variant of Castleman disease is associated with paraneoplastic pemphigus: a 20-year retrospective study of 123 Chinese patients with Castleman disease. Am J Clin Pathol 154(3):403–413
Malik AM, Tupchong S, Huang S et al (2021) An updated review of pemphigus diseases. Medicina. 57(10):1080
Svoboda SA, Huang S, Liu X et al (2021) Paraneoplastic pemphigus: revised diagnostic criteria based on literature analysis. J Cutan Pathol 48(9):1133–1138
Van Rhee F, Wong RS, Munshi N et al (2014) Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol 15(9):966–974
van Rhee F, Rosenthal A et al (2022) Siltuximab is associated with improved progression-free survival in idiopathic multicentric Castleman disease. Blood Adv 6(16):4773–4781
Zhang L, Zhao AL, Duan MH et al (2019) Phase 2 study using oral thalidomide-cyclophosphamide-prednisone for idiopathic multicentric Castleman disease. Blood. 133(16):1720–1728
Zhang L, Zhang M, Cao X et al (2022) A prospective, multicenter study of bortezomib, cyclophosphamide, and dexamethasone in relapsed/refractory iMCD. Leuk Lymphoma 63(3):618–626
Shin D, Jeon YK, Hong YS et al (2011) Clinical dissection of multicentric Castleman disease. Leuk Lymphoma 52(8):1517–1522
Melikyan AL, Egorova EK, Kovrigina AM et al (2015) Clinical and morphological features of different types of Castleman’s disease. Ter Arkh 87(7):64–71
Dispenzieri A, Armitage JO, Loe MJ et al (2012) The clinical spectrum of Castleman’s disease. Am J Hematol 87(11):997–1002
Seo S, Yoo C, Yoon DH et al (2014) Clinical features and outcomes in patients with human immunodeficiency virus-negative, multicentric Castleman’s disease: a single medical center experience. Blood Res 49(4):253–258
Guo M, Lin N, Wang M et al (2022, PREPRINT (Version 1) available at Research Square) A real-world study on a large retrospective cohort of Castleman disease from a single center. https://doi.org/10.21203/rs.3.rs-1748759/v1
Acknowledgements
We thank all the authors and participants for their contributions to the trial. We thank Dr. Nong L and her team for reviewing the pathological slides.
Funding
This study was supported by Beijing Natural Science Foundation No. 7232175.
Author information
Authors and Affiliations
Contributions
GMY collected, analyzed, and interpreted data and co-wrote the manuscript. NL and her team performed the pathological analysis and co-wrote the manuscript. WMY, ZY, WLH, SYH, WQY, LHH, OJP, CXN, and RHY participated in the collection and interpretation of data. DYJ designed and co-wrote the manuscript and coordinated and supervised the project. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• A large cohort of CD from a single center were reclassified and re-evaluated according to the 2017 CDCN diagnostic criteria.
• In the real-world setting, UCD patients complicated with PNP and MCD have poor prognosis even after intensive therapeutic treatments.
Supplementary information
ESM 1
Supplement Tab.1 Treatment and deaths of UCD patients, Supplement Tab.2 Treatment and deaths of MCD patients, Supplement Tab.3 Different chemotherapy regimens and cycles of MCD patients, Supplement Fig.1 Proportion of regions affected by UCD, Supplement Fig.2 Representative pathological images of different subtype of UCD and MCD. (DOCX 358 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, M., Nong, L., Wang, M. et al. Retrospective cohort evaluation of non-HIV Castleman disease from a single academic center in Beijing, China. Ann Hematol 103, 153–162 (2024). https://doi.org/10.1007/s00277-023-05472-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05472-3