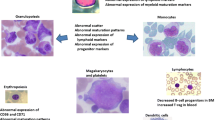
Abstract
Diagnosis and prognostic stratification of myelodysplastic syndromes (MDS) have been complemented by new techniques, including flow cytometry and NGS. To analyze the relationship between molecular and cytofluorimetric data, we enrolled in this retrospective study, 145 patients, including 106 diagnosed with MDS and 39 controls. At disease onset, immunophenotypic (IF), cytogenetic tests, and cytomorphological (CM) examination on bone marrow were carried out in all patients, while NGS was performed in 58 cases. Ogata score presented a specificity of 100% and a sensitivity of 59%. The detection of at least two phenotypic aberrancies in Ogata negative patients increased the sensitivity to 83% and specificity to 87%. Correlations were identified between IF aberrancies and mutations, including positive Ogata>2 and mutations in SRSF2 (p=0.035), CD15 and U2AF1 (0.032), CD56 and DNMT3A (p=0.042), and CD38 and TP53 (p=0.026). In multivariate analysis, U2AF1 mutations, associated with del(20q) and/or abnormalities of chromosome 7 (group 4 as defined by the EuroMDS score), significantly correlated with an inferior overall survival (p=0.019). These parameters and Ogata score>2 also showed a significant correlation with inferior event-free survival (p=0.023 and p=0.041, respectively). Both CM and FC features correlated with prognosis and mutational patterns. In an integrated MDS work-up, these tools may guide indications for mutational screening for optimal risk stratification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal disorders characterized by persistent peripheral cytopenia, dysplasia of myeloid progenitors, and increased risk of acute myeloid leukemia (AML) [1]. The backbone for establishing diagnosis and prognosis of MDS are a careful anamnesis (to exclude potential other causes of cytopenia), bone marrow (BM), cytomorphological (CM) examination, and cytogenetic tests [2].
Over the years, these valuable tools have been complemented by new techniques for a better characterization and prognosis assessment of MDS. In this line in 2014, the European Leukemia Net (ELN) working group for flow cytometry strongly recommended the use of this technique in the work-up of MDS [3].
Despite the lack of a standardized profile for diagnosis, flow cytometry (FC) has been shown to be reliable in enumerating bone marrow progenitors and CD34+ cells and identifying phenotypic aberrancies, which correlate with transfusion dependence, poor response to therapy, unfavorable cytogenetics, evolution into AML, and decreased overall survival (OS) [3,4,5,6]. In clinical practice, a FC score has been proposed by Ogata et al. in 2009 [7] and validated by Della Porta et al. in 2012 [8] for IPSS low-risk MDS, whose diagnosis may be challenging in absence of blasts, ring sideroblasts, or clonal cytogenetic abnormalities.
More recently, the advances in genome sequencing have led to a better understanding of the genomic landscape of MDS, detecting mutations in 78–89% of cases [9,10,11,12]. These acquisitions have allowed to create new classifications and predict more accurately response to treatment and prognosis.
In particular, the recently published International Molecular Prognosis Scoring System (IPSS-M) [13], combining genomic profiling with hematologic and cytogenetic parameters, improved risk stratification of patients with primary and secondary or therapy-related MDS. Indeed, by stratifying MDS into 6 risk classes, IPSS-M predicts survival with significantly higher accuracy, as compared to IPSS [14] and revised IPSS (R-IPSS) [15, 16].
Despite these advances, the relationship between molecular and FC data and the diagnostic potential of the combined analysis need to be defined.
In this retrospective study, we propose a FC panel to detect phenotypic aberrancies on CD34+ myeloid progenitors, in combination with the “Ogata score,” to support the diagnosis and prognosis of MDS and explore the correlations with morphologic examination and mutational profile.
Patients and methods
Patient characteristics
This retrospective study included 145 patients consecutively diagnosed with MDS from November 2017 to February 2022 at Policlinico Universitario di Roma Tor Vergata and Policlinico Universitario di Roma Umberto I (106 with MDS and 39 with non-clonal cytopenias, as controls). Diagnosis of MDS was made according to 2016 WHO criteria [1]. Patient characteristics are summarized in Table 1.
For each case, potential causes of cytopenia were ruled out, including vitamin or iron deficiency, liver or kidney failure, alcohol intake, heavy metal poisoning, chronic inflammatory diseases, thyroid dysfunction, infectious diseases, immunological or rheumatological causes, autoimmune cytopenias, drug-induced cytopenias, and other hematological disorders.
The threshold to define morphological dysplasia was at least 10% cells of one or more myeloid lineage (erythroid, granulocytic, and megakaryocytic). The R-IPSS and IPSS-M were used to categorize MDS from a prognostic perspective [13, 15]. At the onset of disease, immunophenotypic (IF), cytogenetic tests, and morphologic examination on bone marrow (BM), according to current indications [17,18,19], were carried out on every sample. A subset of 58 patients underwent next-generation sequencing (NGS).
MDS were defined “lower-risk” when R-IPSS was from very low, to intermediate risk, and IPSS-M from very low to intermediate-low. Higher-risk MDS included high and very high risk by R-IPSS, and intermediate-high to very high risk by IPSS-M.
Patients were also categorized basing on the classification proposed by Bersanelli et al. [12] (EuroMDS score): Group 1, MDS with SF3B1 mutations and co-existing mutations in other genes (ASXL1 and RUNX1); Group 2, MDS with TP53 mutations and/or complex karyotype; Group 3, MDS with SRSF2 and concomitant TET2 mutations; Group 4, MDS with U2AF1 mutations associated with deletion of chromosome 20q and/or abnormalities of chromosome 7; Group 5, MDS with SRSF2 mutations with co-existing mutations in other genes (ASXL1, RUNX1, IDH2, and EZH2); Group 6, MDS with isolated SF3B1 mutations (or associated with mutations of TET2 and/or JAK/STAT pathways genes); Group 7, MDS with AML-like mutation patterns (DNMT3A, NPM1, FLT3, IDH1, and RUNX1 genes); and Group 0, MDS without specific genomic profiles.
Flow cytometry
Ogata score was performed as described by Della Porta et al. [8] by analysing the following parameters: (1) lymphocyte to myeloblast CD45 ratio (mean fluorescence intensity [MFI] of CD45 on lymphocytes ÷ MFI of CD45 on CD34+myeloblasts); (2) granulocyte to lymphocyte SSC peak channel ratio (SSC channel number where the maximum number of CD10– granulocytic cells occurs ÷ SSC channel number where the maximum number of lymphocytes occurs); (3) the percentage of CD34+ B-progenitor-related cells in all CD34+ cells; and (4) the percentage of CD34+ myeloblasts in all nucleated cells. One point was given for each parameter outside the normal values. The analysis of phenotypic aberrancies was performed on gated CD34+ cells evaluating: abnormal expression of CD200 and CD25, lack or reduced expression of CD117, HLA-DR, CD33, CD38, and CD13; asynchronous expression of CD15 and CD64; and cross lineage expression of CD7, CD2, CD5, and CD56 (Supplementary table 1). Supplementary table 2 shows the monoclonal antibody panels used for immunophenotypic testing.
Molecular screening
Bone marrow mononuclear cells (BM-MNC) were isolated from diagnostic samples of MDS patients by Ficoll gradient centrifugation using lympholyte-H (Cedaralane).
DNA samples were extracted using the QIAamp DNA Mini Kit (Qiagen AG, Milan, Italy), in accordance with the manufacturer’s instructions and quantified using a Qubit Fluorometer (Life Technologies). DNA samples for NGS screening were processed and analyzed as previously reported [20]. In brief, NGS screening for common somatic mutations in thirty genes known to be involved in MDS pathogenesis (supplementary table 3) was performed according to the commercial Myeloid Solution by SOPHiA GENETICS (SOPHiA GENETICS, Saint-Sulpice, Switzerland) on a MiniSeq® sequencing platform (Illumina, San Diego, California). The NGS analysis was performed on generated FASTQ sequencing files using the SOPHiA DDM® platform that allows for detection, annotation, and pre-classification of genomic mutations (SNVs and Indels) through its SOPHiA™ artificial intelligence. Reads were aligned to the human reference genome (hg19 assembly). Only mutations with a VAF ≥ 2% (variant allele frequency), threshold coverage ≥ 1000x, and identified as highly or potentially pathogenic by the SOPHiA DDM® platform were considered for all subsequent steps of the analysis. Single-nucleotide polymorphisms (SNP), variants localized in the intronic and UTR regions, and synonymous variants were also excluded from the analysis. Targeted-NGS sequencing data are stored at https://www.sophiagenetics.com (SOPHiA DDM platform) and can be extracted using the Sophia-DDM-v4 password-protected software. Raw data will be provided to researchers upon request to the corresponding author.
Statistical analysis
Univariate and multivariate analyses were used to establish the connections between the variables. Categorical variables were compared using the chi-square test; odds ratios (OR) with 95% confidence intervals (CI) were also calculated. For expected cell values less than 5, Fisher’s exact test and the exact limits for confidence intervals were preferred. The independent test or Mann-Whitney test was used for continuous variables as appropriate. A p value less than 0.05 was considered significant. Sensitivity [true positives (TP)/TP + false negatives (FN)] and specificity [true negatives (TN)/TN + false positives (FP)] were used to determine the diagnostic power and reliability of the scores.
Survival functions were computed with the Kaplan-Meier method; significance was established with log-rank test. OS was defined as survival from date of first diagnosis, while event-free survival (EFS) indicated time from initial diagnosis and disease progression to HR-MDS, AML, or death by any cause. Cox regression was used to determine significant independent prognostic factors affecting survival. Statistical analysis was performed through IBM SPSS Statistics 27 (IBM Corp. in Armonk, NY).
Results
Flow cytometry and morphology
In this paper, we show that Ogata score >2 was able to identify 63 of 106 MDS patients without misclassifying any control, resulting in 59% sensitivity and 100% specificity. The analysis of CD34+ cells revealed a median of two phenotype aberrations per patient (range 0–7, supplementary fig. 1), with lack of CD33 expression being the most commonly detected (44%, Supplementary fig. 2).
In the subset of patients with a negative Ogata score (score 0–1, 43/106 MDS, 40%), at least 2 aberrancies, among those investigated in the FC panel (as listed in supplemental table 1), were detected in 58% of cases (25/43). This increased the sensitivity of the combined analysis to 83% (TP 88/106), and the specificity to 87% (true negative, TN 34/39).
The sensitivity of Ogata score, in the subgroup of lower-risk MDS, was 51% and 47.5% (true positive, TP: 44/86 and 19/40, respectively), while, as expected, sensitivity in higher risk MDS was 100% and 78% (TP 19/19 and 7/9, according to R-IPSS and IPSS-M, respectively.
Moreover, in Ogata score ≤1 lower-risk MDS (R-IPSS: 42/86, 49%; IPSS-M 21/40, 52.5%) the detection of at least 2 phenotypic aberrancies markedly increased the reliability of the tool, bringing the sensitivity to 79% (R-IPSS, 24/42 patients) and 75% (IPSS-M, 11/21 patients), despite lowering specificity to 87% in both groups (TN 34/39).
The mean FC blast count (CD34+ blasts) was 2.2% (median 0.9, range 0–12). We identified a correlation between higher blast counts and specific FC aberrancies (CD38, p=0.002, R=0.357; CD56, p=0.024, R=0.101) and Ogata score ≥2 (p<0.001, R=0.424) (Supplementary fig. 3).
To assess the predictive role of FC in our patient cohort, we performed an outcome analysis, which revealed a correlation between Ogata score ≥2 and higher FC blast count with AML evolution (p=0.001 and p=0.024, respectively), shorter OS (p=0.004 and p=0.002, respectively), and event-free survival (p=0.003 and p=0.001, respectively). Furthermore, we found a correlation with higher R-IPSS (both p<0.001), while IPSS-M did not reach the significance threshold.
CM blast count was performed in 92 patients (mean blast count 3.1; median 2, range 0–15). Higher blast counts correlated with specific FC aberrancies (CD15, p=0.024, R=0.271; CD56, p=0.006, R=0.156), and a positive Ogata score (p=0.007, R=0.338) (Supplementary fig. 3), progression to AML (p=0.001), shorter EFS (p<0.001), OS (p<0.001), and higher R-IPSS (p<0.001), but not with IPSS-M.
Blast enumerations through FC and CM were strongly correlated (p<0.001). We then compared the two techniques subgrouping the patients according to blast counts (group 1: <5% blasts, group 2: ≥5% and <10%, and group 3: ≥10%). Using FC, 78 (85%) patients were assigned to group 1, 9 (10%) to group 2, and 5 (5%) to group 3, while using CM, 71 (77%) patients were assigned to group 1, 15 (16%) to group 2, and 6 (6%) to group 3. The concordance index was 81.5% (Supplementary fig. 4).
Mutational screening
Using NGS, we detected a total of 81 pathogenic/likely pathogenic mutations in 38 out of 58 patients, (65.5%; median number of mutation per patient: 1, range 0–6) (Supplementary table 4). The median number of mutated genes per patient was 1 (range 0–5).
The most frequently mutated genes were SF3B1 (17%, 10/58 patients), ASXL1 (14%, 8/58 patients), and TET2 (14%, 8/58 patients). Figure 1 shows the distribution of mutations.
In lower risk R-IPSS, 33 out of 53 patients (62%) presented at least 1 mutation, while in R-IPSS higher-risk subgroups all 4 patients were mutation carriers (100%; p=ns). As previously described [9], the detection of a high number of mutations (≥2) correlated with shorter OS (p=0.009) and EFS (p=0.056) (suppl. Fig. 5).
We then analyzed the subgroups of patients with mutation in splicing (SF3B1, SRSF2, U2AF1, and ZRSR2; 21/58 patients, 36%) and epigenetics modifying genes (DNMT3A, IDH1, IDH2, and TET2; 16/58 patients, 27.5%). The former presented significantly lower OS (p=0.004) and EFS (p=0.005).
Supplementary table 4 shows the genomic groups according to the classification proposed by Bersanelli et al. [12]. The most frequently represented was group 0 (MDS without specific genomic profiles; 48%) in agreement with the high prevalence of low-risk-MDS. Group 4 (MDS with U2AF1 mutations associated with deletion of chromosome 20q and/or abnormalities of chromosome 7) presented shorter OS (p<0.001) and EFS (p<0.001), as previously reported [12].
Correlation between flow cytometry and mutational screening
The detection of >2 mutations correlated with Ogata score ≥2 (p=0.001, OR 6.750; 95% CI: 2.048–22.250) (Supplementary fig. 6), but not with BM blast counts assessed by CM. Number of FC aberrancies did not correlate with number of mutations. However, there were significant associations between FC and genomic data (gene mutations and molecular/prognostic subgroups defined according to Bersanelli et al. [12]).
Ogata score ≥2 was found to be significantly associated with the presence of epigenetic modifier genes as well as SRSF2 and TET2 mutations (p=0.003, p=0.035, and p=0.001, respectively), whereas Ogata score <2 was found to be significantly associated with EuroMDS group 0 (MDS without specific genomic profiles, p=0.009). In terms of FC aberrancies, CD56 expression was associated with DNMT3A mutation and EuroMDS group 7 (p=0.042 and p=0.023, respectively), CD15 expression was associated with U2AF1 mutation and EuroMDS group 4 (p=0.032 for both), lower CD117 expression was associated with EuroMDS group 2 (p=0.052), and CD38 aberrancy was associated with TP53 mutation (p=0.026) (Table 2). A high CM blast count correlated with TP53 mutation (p=0.053, R=0.439) and group 2 (MDS with TP53 mutations and/or complex karyotype; p<0.001, R=0.561) whereas a high FC blast count correlated with RUNX1 mutation (p=0.024, R=0.266) and TP53 mutations (p=0.041, R=0.315).
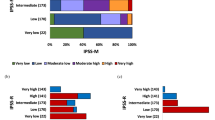
Finally, in multivariate analysis (taking into account age, Ogata score≥2, detection of >2 mutations, CM and FC blast count, mutational subgroup 4 and splicing genes mutations), EuroMDS score group 4 resulted significantly associated with shorter OS (p=0.019). Multivariate analysis of EFS (taking into account age, Ogata score≥2, CM and FC blast count, mutational subgroup 4, and splicing genes mutations) showed a correlation between group 4 and Ogata score ≥2 (p=0.023 and p=0.041 respectively) (Fig. 2).
Discussion
The Ogata score provides high specificity when combined with morphology and cytogenetics, as evidenced by our experience (100% specificity). Since its first formulation in 2009, attempts have been made to implement the sensitivity of Ogata score. In Ogata's own paper, a score extended to 7 parameters (CD15, CD56, and CD11b expression on CD34+ myeloblasts) was proposed, which conferred good diagnostic power (sensitivity 72–86%, specificity 90–98%) [7], but it was not validated in a subsequent trial [8].
Other authors proposed the implementation of specific PAs [21, 22] or more complex indices, such as Ki-67 [23], or the study of erythroid precursors [24, 25] or mast-cells [26], with variable results.
Recently, ELN recommended a second-level panel to investigate myeloid progenitor in MDS. Our panel, which differed slightly from the one recommended by ELN [27] due to the inclusion of CD2 and the exclusion of CD11b (see supplementary table 2), confirmed in a real-world setting the value of adding FC parameters to the Ogata score, providing an improvement in sensitivity (83%).
In this regard, specific PAs have been investigated as prognostic factors. Ogata et al., since the early 2000s, identified in several papers CD117, expressed by myeloid progenitors, and CD56 and CD7, usually expressed in other-than myeloid lineages, as markers of higher blast count, evolution to AML and poor prognosis. On the other hand, CD15, whose expression begin to appear in promyelocytes and myelocytes, correlated with a lower blast count and better outcome. In this line, the authors suggested that a more immature immunophenotype could predict an adverse outcome [28,29,30].
Their interpretation has been questioned in subsequent years, since conflicting evidence about the prognostic role of CD7 emerged: Satoh et al. and Veltroni et al. confirmed the poor prognosis linked to CD7 expression [30, 31], whereas Font et al., analyzing CD34 myeloid cells from 55 bone marrows of patients with MDS, did not detect nor its prevalent expression in higher-risk MDS, neither an association with IPSS or clinical outcome [32, 33].
In our experience, we found a correlation between Ogata Score <2 and Euroscore group 0, that is patients without a specific genetic profile, suggesting that these patients are neither molecularly nor phenotypically well defined, and therefore their characterization remains an unmet medical need. This also questions the diagnosis of MDS in at least some of these patients. In this line, the importance of careful differential diagnosis between MDS, in particular low-risk subtypes, and vitamin deficiencies or cytopenia related to other conditions emerges even more.
The study of potential aberrancies beyond Ogata Score in this setting could be of great help in clarifying the diagnosis and guiding therapeutic. Actually, in our cohort, the detection of 2 phenotypic aberrancies, any of those included in the panel used, resulted highly informative and improved sensitivity in Ogata score-negative patients. In parallel, a broader and more comprehensive phenotypic examination may have a central role in characterize and distinguish pre-malignant conditions, such as idiopathic cytopenia of undetermined significance (ICUS) and clonal cytopenia of undetermined significance (CCUS), in which, again, Ogata Score may not be informative [34]. Further studies are needed to clarify this aspect.
Furthermore, we confirmed the prognostic impact of CD56, as a marker associated with higher blast counts (by both CM and FC analysis) and AML-like mutations patterns (Group 7 and DNMT3A mutations), and the correlation between lower CD117 expression and Bersanelli group 2, characterized by dismal outcome [12], while we did not find any correlations between CD7 and adverse outcome, according to Font et al.
Of note, CD15 expression on CD34+ cells not only did not predict a better outcome in our study cohort, but correlated to higher CM blast counts and to a poor prognosis mutational pattern (U2AF1 mutation and group 4) [12].
Our data confirmed the prognostic value of Ogata Score which, in multivariate analysis, resulted an independent factor significantly associated to EFS.
The comparison between CM examination and FC in blast enumeration is at center of a long-lasting debate. Both techniques are affected by operator dependence and by variables related to blood collection and sample processing. Nevertheless, the strong correlation between the two techniques with a concordance index of 81.5% in blast enumeration found in our study is consistent with previous studies [4]. On the other hand, data in literature about the comparison between FC and CM examination, which, in our experience, tended to identify a higher blast number, are conflicting [4, 35].
Possible explanations could lie in the heterogenicity of the study cohorts and interoperator variability.
The NGS analysis confirmed the negative prognostic role of a high number of mutations, the presence of mutations belonging to splicing-machinery pathway and group 4 identified by Bersanelli et al. [12], whose independent association with OS and EFS is retained, despite taking into account morphological and cytofluorimetric parameters in multivariate analysis. Besides its prognostic value, NGS has been shown to play a role in predicting post hematopoietic cell transplantation outcomes [36] and response to treatments [37, 38].
Thus, in recent years, the use of NGS has increased in clinical laboratories, even outside the field of scientific research [39], although unsolved needs remains, such as the establishment of common quality standards and clinical interpretation of the results. In fact, even if position papers have been published [11, 40], wide variations among laboratories still persist [41]. Furthermore, NGS is still a high-cost technique and low-income countries are struggling to adapt [42]. Moreover, its scarce and uneven availability makes its use complicated even in countries with greater resources.
Therefore, it is necessary to identify the correct subset of patients who could benefit the most from NGS test and the proper panel of genes to analyze to reduce costs and turn around times. According to our findings, specific FC characteristics such as Ogata score and specific PAs correlate with mutational patterns, which can predict outcome and treatment response in the majority of patients. As a result, these low-cost first-line tools could help guide the selection of patients for mutational screening for optimal risk stratification.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF et al (2022) The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 36:1703–1719
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391–2405
Porwit A, van de Loosdrecht AA, Bettelheim P, Brodersen LE, Burbury K, Cremers E et al (2014) Revisiting guidelines for integration of flow cytometry results in the WHO classification of myelodysplastic syndromes-proposal from the International/European LeukemiaNet Working Group for Flow Cytometry in MDS. Leukemia 28:1793–1798
Kern W, Haferlach C, Schnittger S, Haferlach T (2010) Clinical utility of multiparameter flow cytometry in the diagnosis of 1013 patients with suspected myelodysplastic syndrome: correlation to cytomorphology, cytogenetics, and clinical data. Cancer 116:4549–4563
van de Loosdrecht AA, Westers TM, Westra AH, Dräger AM, van der Velden VHJ, Ossenkoppele GJ (2008) Identification of distinct prognostic subgroups in low- and intermediate-1-risk myelodysplastic syndromes by flow cytometry. Blood 111:1067–1077
Alhan C, Westers TM, van der Helm LH, Eeltink C, Huls G, Witte BI et al (2014) Absence of aberrant myeloid progenitors by flow cytometry is associated with favorable response to azacitidine in higher risk myelodysplastic syndromes. Cytometry B Clin Cytom 86:207–215
Ogata K, Della Porta MG, Malcovati L, Picone C, Yokose N, Matsuda A et al (2009) Diagnostic utility of flow cytometry in low-grade myelodysplastic syndromes: a prospective validation study. Haematologica 94:1066–1074
Della Porta MG, Picone C, Pascutto C, Malcovati L, Tamura H, Handa H et al (2012) Multicenter validation of a reproducible flow cytometric score for the diagnosis of low-grade myelodysplastic syndromes: results of a European LeukemiaNET study. Haematologica 97:1209–1217
Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P et al (2013) Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122:3616–3627 quiz 3699
Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G et al (2014) Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 28:241–247
Palomo L, Ibáñez M, Abáigar M, Vázquez I, Álvarez S, Cabezón M et al (2020) Spanish Guidelines for the use of targeted deep sequencing in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol 188:605–622
Bersanelli M, Travaglino E, Meggendorfer M, Matteuzzi T, Sala C, Mosca E et al (2021) Classification and personalized prognostic assessment on the basis of clinical and genomic features in myelodysplastic syndromes. J Clin Oncol Off J Am Soc Clin Oncol 39:1223–1233
Bernard E, Tuechler H, Greenberg PL, Hasserjian RP, Ossa JEA, Nannya Y et al (2022) Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evid 1:EVIDoa2200008
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079–2088
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F et al (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120:2454–2465
Wu J, Zhang Y, Qin T, Xu Z, Qu S, Pan L et al (2022) IPSS-M has greater survival predictive accuracy compared with IPSS-R in persons ≥ 60 years with myelodysplastic syndromes. Exp Hematol Oncol 11:73
Brito-Babapulle F, Catovsky D, Galton DA (1987) Clinical and laboratory features of de novo acute myeloid leukaemia with trilineage myelodysplasia. Br J Haematol 66:445–450
Goasguen JE, Matsuo T, Cox C, Bennett JM (1992) Evaluation of the dysmyelopoiesis in 336 patients with de novo acute myeloid leukemia: major importance of dysgranulopoiesis for remission and survival. Leukemia 6:520–525
Della Porta MG, Travaglino E, Boveri E, Ponzoni M, Malcovati L, Papaemmanuil E et al (2015) Minimal morphological criteria for defining bone marrow dysplasia: a basis for clinical implementation of WHO classification of myelodysplastic syndromes. Leukemia 29:66–75
Fabiani E, Cicconi L, Nardozza AM, Cristiano A, Rossi M, Ottone T et al (2021) Mutational profile of ZBTB16-RARA-positive acute myeloid leukemia. Cancer Med 10:3839–3847
Bardet V, Wagner-Ballon O, Guy J, Morvan C, Debord C, Trimoreau F et al (2015) Multicentric study underlining the interest of adding CD5, CD7 and CD56 expression assessment to the flow cytometric Ogata score in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Haematologica 100:472–478
Alayed K, Meyerson JB, Osei ES, Blidaru G, Schlegelmilch J, Johnson M et al (2020) CD177 enhances the detection of myelodysplastic syndrome by flow cytometry. Am J Clin Pathol 153:554–565
Mestrum SGC, Cremers EMP, de Wit NCJ, Drent RJM, Ramaekers FCS, Hopman AHN et al (2022) Integration of the Ki-67 proliferation index into the Ogata score improves its diagnostic sensitivity for low-grade myelodysplastic syndromes. Leuk Res 113:106789
Mathis S, Chapuis N, Debord C, Rouquette A, Radford-Weiss I, Park S et al (2013) Flow cytometric detection of dyserythropoiesis: a sensitive and powerful diagnostic tool for myelodysplastic syndromes. Leukemia 27:1981–1987
Cremers EMP, Westers TM, Alhan C, Cali C, Visser-Wisselaar HA, Chitu DA et al (2017) Implementation of erythroid lineage analysis by flow cytometry in diagnostic models for myelodysplastic syndromes. Haematologica 102:320–326
Kárai B, Bedekovics J, Miltényi Z, Gergely L, Szerafin L, Ujfalusi A et al (2017) A single-tube flow cytometric procedure for enhancing the diagnosis and prognostic classification of patients with myelodysplastic syndromes. Int J Lab Hematol 39:577–584
van der Velden VHJ, Preijers F, Johansson U, Westers TM, Dunlop A, Porwit A et al (2023) Flow cytometric analysis of myelodysplasia: pre-analytical and technical issues-recommendations from the European LeukemiaNet. Cytometry B Clin Cytom 104:15–26
Ogata K, Satoh C, Hyodo H, Tamura H, Dan K, Yoshida Y (2004) Association between phenotypic features of blasts and the blast percentage in bone marrow of patients with myelodysplastic syndromes. Leuk Res 28:1171–1175
Ogata K, Nakamura K, Yokose N, Tamura H, Tachibana M, Taniguchi O et al (2002) Clinical significance of phenotypic features of blasts in patients with myelodysplastic syndrome. Blood 100:3887–3896
Satoh C, Tamura H, Yamashita T, Tsuji T, Dan K, Ogata K (2009) Aggressive characteristics of myeloblasts expressing CD7 in myelodysplastic syndromes. Leuk Res 33:326–331
Veltroni M, Sainati L, Zecca M, Fenu S, Tridello G, Testi AM et al (2009) Advanced pediatric myelodysplastic syndromes: can immunophenotypic characterization of blast cells be a diagnostic and prognostic tool? Pediatr Blood Cancer 52:357–363
Font P, Subirá D, Mtnez-Chamorro C, Castañón S, Arranz E, Ramiro S et al (2006) Evaluation of CD7 and terminal deoxynucleotidyl transferase (TdT) expression in CD34+ myeloblasts from patients with myelodysplastic syndrome. Leuk Res 30:957–963
Font P, Subirá D (2008) Expression of CD7 in myelodysplastic syndromes (MDS): is this a truly prognostic factor? Leuk Res 32:185–186
Dimopoulos K, Hansen OK, Sjö LD, Saft L, Schjødt IM, Werner Hansen J et al (2020) The diagnostic and prognostic role of flow cytometry in idiopathic and clonal cytopenia of undetermined significance (ICUS/CCUS): a single-center analysis of 79 patients. Cytometry B Clin Cytom 98:250–258
Huang M, Zhao X, Xu H, Liu S, Wang Z, Sui X et al (2017) Correlation between bone marrow blasts counts with flow cytometry and morphological analysis in myelodysplastic syndromes. Ann Lab Med 37:450–453
Hamilton BK, Rybicki L, Hirsch C, Przychodzen B, Nazha A, Gerds AT et al (2019) Mutation clonal burden and allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant 54:1281–1286
Hunter AM, Komrokji RS, Yun S, Al Ali N, Chan O, Song J et al (2021) Baseline and serial molecular profiling predicts outcomes with hypomethylating agents in myelodysplastic syndromes. Blood Adv 5:1017–1028
Cai L, Zhao X, Ai L, Wang H (2020) Role Of TP53 mutations in predicting the clinical efficacy of hypomethylating therapy in patients with myelodysplastic syndrome and related neoplasms: a systematic review and meta-analysis. Clin Exp Med 20:361–371
Vantyghem S, Peterlin P, Thépot S, Ménard A, Dubruille V, Debord C et al (2021) Diagnosis and prognosis are supported by integrated assessment of next-generation sequencing in chronic myeloid malignancies. A real-life study. Haematologica 106:701–707
Aguilera-Diaz A, Vazquez I, Ariceta B, Mañú A, Blasco-Iturri Z, Palomino-Echeverría S et al (2020) Assessment of the clinical utility of four NGS panels in myeloid malignancies. Suggestions for NGS panel choice or design. PLoS One 15:e0227986
Pine AB, Chokr N, Stahl M, Steensma DP, Sekeres MA, Litzow MR et al (2020) Wide variation in use and interpretation of gene mutation profiling panels among health care providers of patients with myelodysplastic syndromes: results of a large web-based survey. Leuk Lymphoma 61:1455–1464
Anwar N, Memon FA, Shahid S, Shakeel M, Irfan M, Arshad A et al (2021) The Dawn of next generation DNA sequencing in myelodysplastic syndromes- experience from Pakistan. BMC Genomics 22:903
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement. This work was supported by AIRC 5×1000 call “Metastatic disease: the key unmet need in oncology” to MYNERVA project (#21267) (MYeloid Neoplasms Research Venture AIRC). A detailed description of the MYNERVA project is available at http://www.progettoagimm.it, MUR-PNRR M4C2I1.3 PE6 project PE00000019 Heal Italia, and Celgene/BMS (project CL-MDS-PI-13673).
Author information
Authors and Affiliations
Contributions
LG analyzed the data. LG and EF wrote the manuscript. EF, CA, HH, AC, MI, CC, and LM performed the experiments. RL, SF, FB, MTV, and LM enrolled patients. MTV and LM designed the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of the “University of Rome Tor Vergata.”
Consent to participate
Participants gave written consent, according to the Declaration of Helsinki.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 250 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guarnera, L., Fabiani, E., Attrotto, C. et al. Reliability of flow-cytometry in diagnosis and prognostic stratification of myelodysplastic syndromes: correlations with morphology and mutational profile. Ann Hematol 102, 3015–3023 (2023). https://doi.org/10.1007/s00277-023-05384-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05384-2