Abstract
Lenalidomide is a synthetic analog of thalidomide formed by the removal of one keto group (plus the addition of an amino group); it has anti-tumor activities beneficial for the treatment of hematologic malignancies. However, lenalidomide distribution to brain in animal models is reportedly low compared with that of thalidomide. The aim of this study was to evaluate plasma and cerebrospinal fluid concentrations of lenalidomide in three patients with malignant hematologic malignancies. Lenalidomide was detected in plasma from the three Japanese patients 1.5 h following oral administration of 20 mg lenalidomide using liquid chromatography/mass spectrometry, despite the in vitro gastrointestinal permeability of lenalidomide being low. Clinically observed cerebrospinal fluid-to-plasma ratios of lenalidomide were low (1.3–2.4%). Observed influx permeability values for lenalidomide in monkey blood–brain barrier model and human placental cell systems were one order of magnitude lower than those of thalidomide and another second-generation drug, pomalidomide along with a positive permeability control, caffeine. Because of the low cell-barrier permeability of lenalidomide demonstrated in in vitro assays, clinically relevant pharmacokinetic profiles of lenalidomide resulted in low penetrability from plasma into cerebrospinal fluid in patients with hematologic malignancies. Lenalidomide is conclusively suggested to expert its favorable immunomodulatory effects via systemic exposures in the patients.



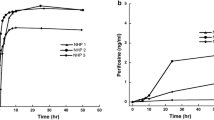
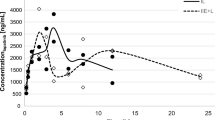
taken from three patients (Table 2). Plots and bars represent means and standard deviations from triplicate determinations
Similar content being viewed by others
References
Kim Y, Schmidt-Wolf IG (2015) Lenalidomide in multiple myeloma ExpertRevAnticancer Ther 15:491–497
Chen N, Zhou S, Palmisano M (2017) Clinical Pharmacokinetics and pharmacodynamics of lenalidomide. Clin Pharmacokinet 56:139–152
Zeldis JB, Carter TL, Knight RD, Hui J (2013) Pomalidomide is teratogenic in rats and rabbits and can be neurotoxic in humans. ProcNatlAcadSciUSA 110:E4819
Benboubker L, Dimopoulos MA, Dispenzieri A et al (2014) Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med 371:906–917
Chanan-Khan AA, Lonial S, Weber D et al (2012) Lenalidomide in combination with dexamethasone improves survival and time-to-progression in patients >/=65 years old with relapsed or refractory multiple myeloma. IntJHematol 96:254–262
Weber DM, Chen C, Niesvizky R et al (2007) Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 357:2133–2142
Dimopoulos M, Spencer A, Attal M et al (2007) Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357:2123–2132
Huang YJ, Liao JF, Tsai TH (2005) Concurrent determination of thalidomide in rat blood, brain and bile using multiple microdialysis coupled to liquid chromatography. Biomed Chromatogr 19:488–493
Muscal JA, Sun Y, Nuchtern JG et al (2012) Plasma and cerebrospinal fluid pharmacokinetics of thalidomide and lenalidomide in nonhuman primates. Cancer Chemother Pharmacol 69:943–947
Rozewski DM, Herman SE, Towns WH 2nd et al (2012) Pharmacokinetics and tissue disposition of lenalidomide in mice. AAPS J 14:872–882
Anwer S, Collings F, Trace K, Sun Y, Sternberg A (2013) Cerebrospinal fluid penetrance of lenalidomide in meningeal myeloma. Br J Haematol 162:281–282
Warren KE, Goldman S, Pollack IF et al (2011) Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium study PBTC-018. J Clin Oncol 29:324–329
Chen N, Kasserra C, Reyes J, Liu L, Lau H (2012) Single-dose pharmacokinetics of lenalidomide in healthy volunteers: dose proportionality, food effect, and racial sensitivity. Cancer Chemother Pharmacol 70:717–725
Chen N, Wen L, Lau H, Surapaneni S, Kumar G (2012) Pharmacokinetics, metabolism and excretion of [(14)C]-lenalidomide following oral administration in healthy male subjects. Cancer Chemother Pharmacol 69:789–797
Chowdhury G, Shibata N, Yamazaki H, Guengerich FP (2014) Human cytochrome P450 oxidation of 5-hydroxythalidomide and pomalidomide, an amino analogue of thalidomide. Chem Res Toxicol 27:147–156
Artursson P, Palm K, Luthman K (2001) Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev 46:27–43
Neuhoff S, Ungell AL, Zamora I, Artursson P (2005) pH-Dependent passive and active transport of acidic drugs across Caco-2 cell monolayers. Eur J Pharm Sci 25:211–220
Neuhoff S, Ungell AL, Zamora I, Artursson P (2003) pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharm Res 20:1141–1148
Nishiyama S, Suemizu H, Shibata N, Guengerich FP, Yamazaki H (2015) Simulation of human plasma concentrations of thalidomide and primary 5-hydroxylated metabolites explored with pharmacokinetic data in humanized TK-NOG mice. Chem Res Toxicol 28:2088–2090
Shimizu M, Suemizu H, Mitsui M, Shibata N, Guengerich FP, Yamazaki H (2017) Metabolic profiles of pomalidomide in human plasma simulated with pharmacokinetic data in control and humanized-liver mice. Xenobiotica 47:844–848
Murayama N, Suemizu H, Uehara S et al (2018) Association of pharmacokinetic profiles of lenalidomide in human plasma simulated using pharmacokinetic data in humanized-liver mice with liver toxicity detected by human serum albumin RNA. J Toxicol Sci 43:369–375
Kamiya Y, Takaku H, Yamada R et al (2020) Determination and prediction of permeability across intestinal epithelial cell monolayer of a diverse range of industrial chemicals/drugs for estimation of oral absorption as a putative marker of hepatotoxicity. Toxicol Rep 7:149–154
Kamiya Y, Omura A, Hayasaka R et al (2021) Prediction of permeability across intestinal cell monolayers for 219 disparate chemicals using in vitro experimental coefficients in a pH gradient system and in silico analyses by trivariate linear regressions and machine learning. Biochem Pharmacol 192:114749
Murayama N, Kazuki Y, Satoh D et al (2017) Induction of human cytochrome P450 3A enzymes in cultured placental cells by thalidomide and relevance to bioactivation and toxicity. J Toxicol Sci 42:343–348
Watanabe D, Nakagawa S, Morofuji Y et al (2021) Characterization of a primate blood-brain barrier co-culture model prepared from primary brain endothelial cells, pericytes and astrocytes. Pharmaceutics 13:1484
Kamiya Y, Handa K, Miura T et al (2021) In silico prediction of input parameters for simplified physiologically based pharmacokinetic models for estimating plasma, liver, and kidney exposures in rats after oral doses of 246 disparate chemicals. Chem Res Toxicol 34:507–513
Kamiya Y, Otsuka S, Miura T et al (2019) Plasma and hepatic concentrations of chemicals after virtual oral administrations extrapolated using rat plasma data and simple physiologically based pharmacokinetic models. Chem Res Toxicol 32:211–218
Kamiya Y, Otsuka S, Miura T et al (2020) Physiologically based pharmacokinetic models predicting renal and hepatic concentrations of industrial chemicals after virtual oral doses in rats. Chem Res Toxicol 33:1736–1751
Gangatharan SA, Carney DA, Prince HM et al (2012) Emergence of central nervous system myeloma in the era of novel agents. Hematol Oncol 30:170–174
Rubenstein JL, Geng H, Fraser EJ et al (2018) Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv 2:1595–1607
Acknowledgements
The authors thank Izumi Sano, Riku Hayasaka, and Makiko Shimizu for their assistance, and David Smallbones for copyediting a draft of this article.
Funding
This study was supported partly by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (20K07139), the Ministry of Health, Labour and Welfare Grant-in-aid for Scientific Research (20KC2008), and the METI Artificial Intelligence-based Substance Hazard Integrated Prediction System Project, Japan.
Author information
Authors and Affiliations
Contributions
DO and HY designed the study. DO, SS, RS, and SM monitored the patients and carried out acquisition of the patient data. NM, YK, and RS conceived the drug monitoring and in vitro permeability assays. HY drafted the manuscript. DO, KT, and KA analyzed the patient medical data and helped to draft the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were carried out in accordance with the ethical standards of the relevant institutional and/or national research committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The research was approved by the Ethical Committees of Tokai University School of Medicine (No. 20R-059) and Isehara Kyodo Hospital (No. 139). Informed consent was obtained from the patient.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ogiya, D., Murayama, N., Kamiya, Y. et al. Low cerebrospinal fluid-to-plasma ratios of orally administered lenalidomide mediated by its low cell membrane permeability in patients with hematologic malignancies. Ann Hematol 101, 2013–2019 (2022). https://doi.org/10.1007/s00277-022-04893-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04893-w