Abstract
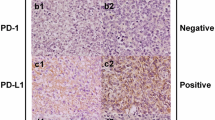
This study aimed to explore the clinicopathological features and prognostic correlation of extranodal natural killer (NK)/T cell lymphoma (ENKTCL) in the early stage, screen out the prognostic markers of ENKTCL, and to establish the molecular model of ENKTCL prognosis. A retrospective study was conducted in 88 patients from May 1999 to Dec 2013 in Chinese Academy of Medical Sciences Cancer Hospital, who were diagnosed with ENKTCL according to WHO lymphoid hematopoietic tumor classification (published in 2008). The clinical data and paraffin-embedded tissue blocks were collected. The expressions of CD56, MLH1, PDGFRA, VEGF, PD-L1, PD-1, CyclinD1, p53, and Ki-67 were detected by high-throughput tissue microarray and immunohistochemistry (IHC) staining. The relationship between nine protein expressions and the clinicopathological features and prognosis of patients with ENKTCL were analyzed. The survival time of the 42 patients with complete clinical and follow-up data was 0~153 months. The average survival time was 60.1 months. The survival rates of 1 year, 2 years, and 3 year were 85.7%, 78.6%, and 71.4%, respectively. Single factor survival analysis showed that the increase of serum lactate dehydrogenase (LDH ≥ 240UI/L) before treatment was associated with poor prognosis, and there was a significant difference in survival rate (P = 0.006). Different therapy methods were related with prognosis (P = 0.011); in specifically, radiotherapy alone had the best treatment effect, followed by concurrent chemoradiotherapy, and the worst was chemotherapy alone. But, multivariate statistics indicated that the LDH level and the treatment approach were not independent prognostic factors of ENKTCL. There was no statistical difference between epidemiological factors such as gender, age, and other clinicopathological factors including tumor location, B symptoms, β2-microglobulin levels before treatment, and prognosis. Survival analysis of single factor showed that the positive expression of PDGFRA and PD-L1 was, respectively, related to the poor prognosis of patients with ENKTCL (P = 0.040, 0.007). The patients with Ki-67 overexpression (≥ 50%) had a worse prognosis than those with lower expression (< 50%), and the difference of survival rate between the two groups has statistical significance (P = 0.038). The expression of CD56, MLH1, VEGF, PD-1, CyclinD1, and p53 has no effect on survival rate (P > 0.05). Multivariate survival analysis showed that the expression levels of PDGFRA, PD-L1, and Ki-67 were independent factors in the prognosis of patients with ENKTCL. And the positive expressions of these three proteins were risk factors for prognosis of patients with ENKTCL (PDGFRA: P = 0.045, HR = 8.265, 95% CI: 1.050–65.054; PD-L1: P = 0.005, HR = 9.369, 95% CI: 1.950–45.003; Ki-67: P = 0.023, HR = 3.545, 95% CI: 1.187–10.585). The elevation of serum lactate dehydrogenase (LDH ≥ 240UI/L) before treatment and the treatment approach were associated with poor prognosis, which could be used as adjunct indexes to the prognosis. However, they were not independent factors for the prognosis of patients with ENKTCL. The expressions of PDGFRA, PD-L1, and Ki-67 were independent factors in the prognosis of patients with ENKTCL and these three proteins were risk factors of prognosis. The above markers combined with clinical factors may establish the prognosis model of ENKTCL.


Similar content being viewed by others
References
Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, Kayasut K, Mitarnun W, Khuhapinant A, Bunworasate U, Puavilai T, Bedavanija A, Garcia-Herrera A, Campo E, Cook JR, Choi J, Swerdlow SH (2012) Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and alphabeta, gammadelta, and alphabeta/gammadelta T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol 36(4):481–499
Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO, Liang R, for the International Peripheral T-Cell Lymphoma Project (2009) Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the international peripheral T-cell lymphoma project. Blood 113(17):3931–3937
Zeng LS, Huang WT, Qiu T, Shan L, Guo L, Ying JM, Lyu N, Feng XL (2017) Correlation between the clinicopathological features and prognosis in patients with extranodal natural killer/T cell lymphoma. Chronic Dis Transl Med 3(4):252–259
Asano N, Kato S, Nakamura S (2013) Epstein-Barr virus-associated natural killer/T-cell lymphomas[J]. Best Pract Res Clin Haematol 26(1):15–21
Augoff K, Hryniewicz-Jankowska A, Tabola R (2015) Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett 358(1):1–7
Lu R, Jiang M, Chen Z, Xu X, Hu H, Zhao X, Gao X, Guo L (2013) Lactate dehydrogenase 5 expression in non-Hodgkin lymphoma is associated with the induced hypoxia regulated protein and poor prognosis. PLoS One 8(9):e74853
Hanakawa H, Orita Y, Sato Y, Takao S, Marunaka H, Morishita T, Yamashita Y, Hori Y, Domae S, Inokuchi I, Akagi S, Kondo E, Iwaki N, Motomiya K, Okumura H, Yoshino T, Nishizaki K (2014) Novel and simple prognostic index for nasal natural killer/T-cell lymphoma. Head Neck 36(4):551–556
Niu SQ, Yang Y, Li YY, Wen G, Wang L, Li ZM, Wang HY, Zhang LL, Xia YF, Zhang YJ (2016) Primary site and regional lymph node involvement are independent prognostic factors for early-stage extranodal nasal-type natural killer/T cell lymphoma. Chin J Cancer 35:34
Li H, Wang CS, Wang XD (2014) Meta analysis of treatment for stage IE~IIE extranodal natural killer /T cell lymphomas in China. Asian Pac J Cancer Prev 15(5):2297–2302
Tse E, Kwong YL (2015) Nasal NK/T-cell lymphoma: RT, CT, or both. Blood 126(12):1400–1401
Tse E, Kwong YL (2017) The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol 10(1):85
Yamaguchi M, Suzuki R, Oguchi M, Asano N, Amaki J, Akiba T, Maeda T, Itasaka S, Kubota N, Saito Y, Kobayashi Y, Itami J, Ueda K, Miyazaki K, Ii N, Tomita N, Sekiguchi N, Takizawa J, Saito B, Murayama T, Ando T, Wada H, Hyo R, Ejima Y, Hasegawa M, Katayama N (2017) Treatments and outcomes of patients with Extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol 35(1):32–39
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, Ishizawa K, Maseki N, Itoh K, Usui N, Wasada I, Kinoshita T, Hotta T, Tsukasaki K, Oshimi K (2012) Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: an updated analysis of the Japan clinical oncology group study JCOG0211. J Clin Oncol 30(32):4044–4046
Shi L, Chen S, Yang L, Li Y (2013) The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol 6(1):74
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, Chen L, Drake CG, Topalian SL, Pardoll DM, Pai SI (2013) Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 73(6):1733–1741
Carter L, Fouser LA, Jussif J et al (2002) PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol 32(3):634–643
Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, Cremonesi E, Heberer M, Iezzi G, Lugli A, Terracciano L, Sconocchia G, Oertli D, Spagnoli GC, Tornillo L (2013) Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 49(9):2233–2242
Mamalis A, Garcha M, Jagdeo J (2014) Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res 306(6):511–519
Ghebeh H, Mohammed S, Al-Omair A et al (2006) The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 8(3):190–198
Sznol M, Chen L (2013) Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 19(5):1021–1034
Huang B, Chen L, Bao C et al (2015) The expression status and prognostic significance of programmed cell death 1 ligand 1 in gastrointestinal tract cancer: a systematic review and meta-analysis. Onco Targets Ther 8:2617–2625
Wu P, Wu D, Li L et al (2015) PD-L1 and survival in solid tumors: a Meta-analysis. PLoS One 10(6):e131403
Hamid O, Carvajal RD (2013) Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther 13(6):847–861
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 372(4):311–319
Hawkes EA, Grigg A, Chong G (2015) Programmed cell death-1 inhibition in lymphoma. Lancet Oncol 16(5):e234–e245
Kim WY, Jung HY, Nam SJ, Kim TM, Heo DS, Kim CW, Jeon YK (2016) Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch 469(5):581–590
Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, Takahara M, Kumai T, Ishibashi K, Kosaka A, Aoki N, Oikawa K, Uno Y, Akiyama N, Sado M, Takei H, Celis E, Harabuchi Y, Kobayashi H (2017) Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother 66(7):877–890
Jo JC, Kim M, Choi Y, Kim HJ, Kim JE, Chae SW, Kim H, Cha HJ (2017) Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 96(1):25–31
Board R, Jayson GC (2005) Platelet-derived growth factor receptor (PDGFR): a target for anticancer therapeutics. Drug Resist Updat 8(1–2):75–83
Yan W, Zhang A, Powell MJ (2016) Genetic alteration and mutation profiling of circulating cell-free tumor DNA (cfDNA) for diagnosis and targeted therapy of gastrointestinal stromal tumors. Chin J Cancer 35(1):68
Karsy M, Neil JA, Guan J et al (2015) A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg Focus 38(3):E4
Kurokawa Y, Matsuura N, Kawabata R et al (2014) Prognostic impact of major receptor tyrosine kinase expression in gastric cancer. Ann Surg Oncol 21(Suppl 4):S584–S590
Piccaluga PP, Agostinelli C, Califano A, Carbone A, Fantoni L, Ferrari S, Gazzola A, Gloghini A, Righi S, Rossi M, Tagliafico E, Zinzani PL, Zupo S, Baccarani M, Pileri SA (2007) Gene expression analysis of angioimmunoblastic lymphoma indicates derivation from T follicular helper cells and vascular endothelial growth factor deregulation. Cancer Res 67(22):10703–10710
Huang Q, Snyder DS, Chu P, Gaal KK, Chang KL, Weiss LM (2011) PDGFRA rearrangement leading to hyper-eosinophilia, T-lymphoblastic lymphoma, myeloproliferative neoplasm and precursor B-cell acute lymphoblastic leukemia. Leukemia 25(2):371–375
Huang Y, de Reynies A, de Leval L, Ghazi B, Martin-Garcia N, Travert M, Bosq J, Briere J, Petit B, Thomas E, Coppo P, Marafioti T, Emile JF, Delfau-Larue MH, Schmitt C, Gaulard P (2010) Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood 115(6):1226–1237
Li J, Chen P, Liu W, Xia Z, Shi F, Zhong M (2016) Expression and significance of Ku80 and PDGFR-alpha in nasal NK/T-cell lymphoma. Pathol Res Pract 212(3):204–209
Czyzewska J, Guzinska-Ustymowicz K, Lebelt A et al (2004) Evaluation of proliferating markers Ki-67, PCNA in gastric cancers. Rocz Akad Med Bialymst 49(Suppl 1):64–66
He X, Chen Z, Fu T, Jin X, Yu T, Liang Y, Zhao X, Huang L (2014) Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta-analysis. BMC Cancer 14:153
Kim SJ, Kim BS, Choi CW, Choi J, Kim I, Lee YH, Kim JS (2007) Ki-67 expression is predictive of prognosis in patients with stage I/II extranodal NK/T-cell lymphoma, nasal type. Ann Oncol 18(8):1382–1387
Huang X, Sun Q, Fu H, Zhou X, Guan X, Wang J (2014) Both c-Myc and Ki-67 expression are predictive markers in patients with extranodal NK/T-cell lymphoma, nasal type: a retrospective study in China. Pathol Res Pract 210(6):351–356
Jiang L, Li P, Wang H, Liu J, Zhang X, Qiu H, Zhang B (2014) Prognostic significance of Ki-67 antigen expression in extranodal natural killer/T-cell lymphoma, nasal type. Med Oncol 31(10):218
Funding
This work was supported by Capital Clinical Characteristic Application Research (Z141107002514046) from Beijing Municipal Science & Technology Commission, and Beijing Hope Run special fund (LC2010A10 and LC2014A18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All patients or their guardians provided written informed consent to allow collection of personal data in accordance with the Declaration of Helsinki.
Conflicts of interests
The authors declare that they have no conflicts of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, L., Huang, W., Cao, Z. et al. The correlation of clinicopathological features and prognosis in extranodal natural killer/T cell lymphoma: a report of 42 cases in the early stage. Ann Hematol 98, 1467–1476 (2019). https://doi.org/10.1007/s00277-019-03643-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03643-9