Abstract
Purpose
Saliva distribution over the palatal surface plays an important role in the perception of dry mouth. It is envisaged that non-invasive estimation of the palatal surface area by anthropometric measurements of the head and face can be useful in the assessment of oral dryness. For this purpose, the relationship between the palatal surface area and anthropometric measurements of the head and face was investigated.
Methods
The palatal surface was measured in 51 healthy volunteers using an intra-oral scanner. The distances between anthropometric landmarks of the head and face were determined using an anatomical sliding caliper. Correlations between the palatal surface area and the anthropometric landmarks were investigated.
Results
The median palatal surface area for the total study population was found to be 2120.6 mm2. Virtually, all anthropometric measurements showed significant differences between females and males. Various head and face measurements had a significant correlation with the palatal surface area. However, these correlations disappeared when the participants were stratified based on their sex, with the exception of mandibular length and palatal width in females.
Conclusion
The surface area of the palate correlates with nearly all anthropometric measurements of the head and face included in this study. Yet, the clinical applicability seems limited to females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human saliva is predominantly produced by three pairs of major glands known as parotid, submandibular, and sublingual glands. These glands are responsible for the production of 90% of the volume of saliva [8]. Each of the glands excretes saliva with a unique consistency into the oral cavity via various salivary ducts [14]. The openings of these salivary ducts are located in various intra-oral locations, such as the buccal mucosa for the parotid glands and the floor of the mouth for the sublingual and submandibular glands [14].
After secretion, saliva is distributed over the various intra-oral surfaces, especially during chewing and swallowing [16, 29]. Several studies have explored the thickness of the salivary film covering the teeth and oral mucosa at various intra-oral locations [6, 15, 28]. The salivary film thickness at the anterior part of the palate seems to be relatively thin compared to other intra-oral surfaces [4, 5, 10, 18, 22, 23, 25, 29, 30]. In addition, in patients suffering from hyposalivation, a reduced salivary film thickness at the anterior palate was observed compared to healthy controls [4, 5, 10, 18, 22, 23, 25, 29, 30].
Next to e.g. the salivary volume, the size of the surface area of the intra-oral regions relates to the salivary film thickness. To investigate the surface area of the oral cavity, previous studies used the so-called foil technique; stone models of dental impressions were prepared and covered with aluminium foil. Subsequently, this foil was weighed to deduce the surface area [6, 15, 28]. Despite the fact that this foil technique has been proven to be reproducible [6, 15, 28] some drawbacks were noted as well; adaptation of the foil onto the models without stretching appeared challenging. Besides, it was difficult to fold the foil completely into interdental spaces, and around the labial and buccal vestibular mucosa [6]. Therefore, in a recent study an alternative strategy was explored using cone-beam computed tomography (CBCT) in combination with digital analysis [3]. However, in contrast to the studies which used the foil technique, the CBCT analysis was performed on cadavers [3]. It was found that CBCT analysis had good reliability for measuring various intra-oral surface areas such as the palate, tongue, mucosa, and hard tissues. The studies using the foil technique and the CBCT analysis showed identical results for the palatal surface area (20.1 ± 1.9 vs. 20.0 ± 2.9 cm2) [3, 6]. In the cadaver study, the sizes of several intra-oral surface areas, including the palatal surface area, were related to facial anthropomorphic measurements [3]. Moderate, yet statistically significant correlations were observed between the palatal surface area and the length of the head, as well as the surface area of the tongue and the depth of the head [3].
However, it was postulated that the study was limited by the fact that soft tissues of the cadavers were solidified by their embalmment in a formaldehyde solution which would lead to a suboptimal approximation of the surface areas [3]. For this reason, in the current study, we included living subjects and also applied an intra-oral scanner, which projected a light source on the intra-oral surfaces to be scanned. Then, images captured by imaging sensors are processed by scanning software to produce triangulated point clouds that enable a virtual 3D surface model to be created [7]. A recent study revealed promising results using this scanner, especially for the documentation of palatal soft tissue in terms of shape, colour, and curvature [9]. Therefore, this study was designed to validate the relation between the palate surface area, measured using an intra-oral scanner, and anthropometric measurements of the head and face in living subjects. A relation between the anthropomorphic measurements and the palatal intra-oral surfaces would potentially enable easy approximation of the palatal intra-oral surface area in a chairside medical setting. Approximation of the palatal surface area might be relevant for clinicians investigating the oral cavity, such as dentists and oral maxillofacial surgeons.
Material and methods
Participants
The study was approved by the Ethics Review Committee at the Academic Centre for Dentistry Amsterdam (ACTA; 202065). Volunteers were recruited at ACTA through posters. Eligibility criteria required volunteers to be 18 years or older. Informed written consent was obtained from all volunteers. Data analysis of volunteers was completed anonymously, and only age and sex were registered. The reporting of this study conformed to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement [27].
A priori sample size calculation was performed using G*Power software, version 3.1.9.4 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany); the correlation coefficient of a previous study was used: 0.59 [3], an α of 0.05, and a power of 80%; 20 participants were needed in each group. Because sex differences affect the anthropometric orofacial measures, minimally 40 participants were needed with almost equal numbers of females and males [11, 21, 32].
Measuring the palatal surface area
To measure the palatal surface area, an intra-oral scan of the upper jaw including the palate (the whole hard palate and part of the soft palate) was taken with the TRIOS 3 scanner (3Shape, version 21.3.5, Copenhagen, Denmark). The scanning protocol of the manufacturer was followed when scanning the intra-oral upper jaw area. Scans were digitally saved in Polygon File Format (PLY) files. Subsequently, each PLY object was analysed twice in Meshmixer (Autodesk, San Rafael, CA, USA) by one researcher (ZA). This analysis involved manual separation of the palate by using the vibrating line including the visible fovea palatine as a cutoff for the length of the palate. Besides, all palatal mucosa including the gingiva around the upper teeth was included in the palatal surface (Fig. 1). After segmentation, the palatal surface areas (in mm2) were determined.
Anthropometric measurements
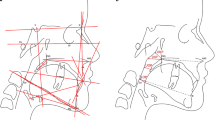
Anthropometric measurements of the head and face were performed as described previously, using the same anthropometric landmarks (see Table 1) [3]. The distance between anthropometric landmarks was determined twice using an anatomical sliding caliper with electronic display showing distance in millimetres (mm). The two measurements were carried out by one researcher (ZA) on the same day.
Statistical analysis
The data were processed in an electronic clinical data-management platform (CastorEdc, Castor, Amsterdam, the Netherlands) and then converted into SPSS, version 27.0 (IBM Corp SPSS Statistics, Armonk, NY, USA) for the statistical analysis. The Shapiro–Wilk test was used to assess the normality of the data. The data were presented as median, and their interquartile range (IQR), as most of the parameters were not normally distributed.
The intraclass correlation coefficient (ICC) was used to determine the degree of agreement between two palatal surface area measurements and between the two anthropometric measurements of the head and face. A two-way mixed, absolute agreement, average-measures ICC was calculated for these measurements [13, 19]. The ICC is indicative of poor (values less than 0.5), moderate (between 0.5 and 0.75), good (between 0.75 and 0.9) and excellent (greater than 0.90) reliability [17].
The mean of the two palatal surface area measurements and the various anthropometric measurements of the head and face were used for further analysis.
Female–male differences for the palatal surface area measurement and the anthropometric measurements of the head and face were explored with the Mann–Whitney U test.
The possible relations between the palatal surface area and anthropometric measurements were analysed with a bootstrapped Spearman rank correlation test (1000 × bootstrapping). The Spearman’s rho coefficient and bias-corrected accelerated (Bca) 95% confidence interval were extracted. Furthermore, the participants were stratified based on their sex. The size of the correlation coefficient was interpreted as poor (r = 0.1–0.2), fair (r = 0.3–0.5), moderate (r = 0.6–0.7) or very strong (r = 0.8–0.9) correlation [1]. All significance levels (p) were set at 0.05.
Results
Fifty-one volunteers signed up for this study: 23 were female and 28 were male (45.1%: 54.9%). The average age was 42.6 ± 14.8 years (range 20–71 years). The average age of female and male participants did not differ significantly (Mann–Whitney U test p > 0.05).
The palatal surface area and anthropometric measurements
The palatal surface area and the anthropometric measurements of the head and face are reported for the total study population as well as the female and male participants separately (Table 2). The median palatal surface area for the total study population was 2120.6 ± 1976.3–2232.0 mm2. The ICC for the palatal surface area measurements and the anthropometric measurements varied between 0.85 and 0.99, which was in the good or excellent range.
Almost all anthropometric measurements showed significant differences between females and males (Mann–Whitney U test p < 0.05), where male participants showed higher values compared to females (Table 2). No sex-related differences were observed for the mouth and palatal widths. There was also no significant difference in the palatal surface area between females and males (Mann–Whitney U test p > 0.05).
Relation between the palatal surface area and anthropometric measurements of the head and face
For the total study population, a significant correlation was found between the palatal surface area and the length of the head, the width of the head, face height, nose height, upper face height, upper lip height, mandibular length and palatal width (Table 3). The correlation coefficients for these correlations varied between 0.29 and 0.37, which indicates poor to fair correlations. These positive correlations indicate that larger dimensions of the head and face are associated with a larger palatal surface area. When the volunteers were stratified based on sex, the female palatal surface area correlated significantly with the mandibular length (0.46) and the palatal width (0.56) (Table 3). These correlations could be considered as fair.
Discussion
This study aimed to assess the possible relation between the dimensions of the palatal surface area and anthropometric measurements of the head and face in living subjects. An intra-oral scanner was used to determine the palatal surface area. The excellent ICC for the palatal surface areas indicated the high reproducibility of the intra-oral scanner technique. Various head and face measurements had a significant correlation with the palatal surface area. When stratified by sex, significant correlations with the female palatal surface were found with the mandibular length and palatal width.
The adult palatal surface area was 2120.6 mm2, which was comparable to findings of other studies with a mean of 1990–2010 mm2 [3, 6, 15]. In these studies, the palatal surface areas were determined using foil impressions taken from stone models [6, 15], while another study used CBCT imaging and digital analysis [3]. Apparently, all methods used so far reveal comparable and representative results, as the reported palatal surface areas are in the same range. In addition, the technique presented in the current study, using an intra-oral scanner, adds up to this line of methods, as these had a very good reproducibility with an excellent ICC. Moreover, the intra-oral scanner has the beneficial effect of not using ionizing radiation and its technique is easy, safe and less laborious.
The palatal surface area in the current study did not differ between the two sexes. This finding is consistent with the results of two other studies [3, 6], while another study revealed that male participants had a significantly larger palatal surface areas compared to females [15]. This latter study, however, included females with a mean age 16.8 ± 8.02 years and males of 20.7 ± 13.4 years old [15]. These participants were considerably younger than the volunteers in the current study with a mean age of 42.6 ± 14.8 years. In this light, is has to be noted that maturation of female facial structures starts at an earlier age compared to males [24]. For this reason, in younger aged groups, there is a significant difference in palatal surface area between the two sexes, which explains why the study by Kerr et al. found significant differences in the palatal surface area measurements [15]. However, when investigating older subjects, such as the current study, these differences in the palatal surface area apparently disappeared.
In the current study, head and face proportions differed significantly between females and males. This finding is broadly supported by the work of other studies describing the effects of sex on anthropometric orofacial measures, mentioning larger measures for males when compared to females [11, 21, 32]. In our previous study, investigating cadavers with CBCT, comparable anthropometric differences between two sexes were observed [3]. In the cadaver study, the length of the head did not differ significantly in the two sexes, while in the current study there was a significant difference in the length of the head between the two sexes. This result could be explained by the limited number of cadavers used in the CBCT study (female N = 8, and male N = 5) [3] compared to the larger number of living subjects in the current study (female N = 23, and male N = 28).
In the current study, various anthropometric measurements had a significant correlation with the palatal surface area. This is in contrast with the CBCT study with human cadavers where only a statistically significant correlation between the length of the head and palatal surface area was observed. There are several possible explanations for this result. Firstly, the previous study included cadavers with possibly solidified soft tissues. Secondly, the number of included subjects might also influence this observation; the cadaver study had a possibly limited statistical power due to the limited number of cadavers used (N = 12). Although in the current study more significant correlations were found between palatal surface area and facial anthropometric measurements, most of these correlations were poor or fair (± 0.3). Finally, sex differences influenced these correlations, as males had significantly larger head and face proportions then females. For this reason, most of the significant correlations disappeared after stratifying the subjects based on their sex, especially for males. Females had a significant correlation between palatal surface with the mandibular length and the palatal width. Possibly, the face type of females attributed to this significant correlation. The face type of females is different compared to males; for females the most common face type is mesoprosop (medium-broad face) or euryprosop (short and wide), while for male it is the leptoprosop (long and narrow) and hyperleptoprosop [2, 31].
Previous studies measured not only the palatal surface, but also palatal volume. This palatal volume can contribute to explore the timing of surgery and surgical protocols [12, 20, 26]. In addition, palatal volume measurements can help to evaluate changes induced by treatment modalities such as rapid palatal expansion and in the orthopaedic treatment of cleft palate cases and to evaluate changes in orthodontic treatment [12, 20, 26]. Therefore, future studies exploring the relation between the palatal volume and anthropometric measurements are also warranted.
Conclusion
An optical scanner was successfully used to determine the palatal surface area, as the ICC for the palatal surface area was in excellent range. Various head and face proportions had a significant correlation with the palatal surface area. When stratified by sex, significant correlations with the female palatal surface were found with the mandibular length and palatal width.
References
Akoglu H (2018) User’s guide to correlation coefficients. Turk J Emerg Med 18:91–93
Arslan SG, Genç C, Odabaş B, Kama JD (2008) Comparison of facial proportions and anthropometric norms among Turkish young adults with different face types. Aesthetic Plast Surg 32:234–242
Assy Z, Klop C, Brand HS, Hoogeveen RC, Koolstra JH, Bikker FJ (2020) Determination of intra-oral surface areas by cone-beam computed tomography analysis and their relation with anthrometric measurements of the head. Surg Radiol Anat 42:1063–1071
Chaudhury NM, Proctor GB, Karlsson NG, Carpenter GH, Flowers SA (2016) Reduced mucin-7 (Muc7) sialylation and altered saliva rheology in Sjögren’s syndrome associated oral dryness. Mol Cell Proteomics 15:1048–1059
Chaudhury NM, Shirlaw P, Pramanik R, Carpenter GH, Proctor GB (2015) Changes in saliva rheological properties and mucin glycosylation in dry mouth. J Dent Res 94:1660–1667
Collins LM, Dawes C (1987) The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J Dent Res 66:1300–1302
Daly S, Seong J, Parkinson C, Newcombe R, Claydon N, West N (2021) A proof of concept study to confirm the suitability of an intra oral scanner to record oral images for the non-invasive assessment of gingival inflammation. J Dent 105:103579
De Paula F, Teshima THN, Hsieh R, Souza MM, Nico MMS, Lourenco SV (2017) Overview of human salivary glands: highlights of morphology and developing processes. Anat Rec (Hoboken) 300:1180–1188
Deferm JT, Schreurs R, Baan F, Bruggink R, MaW M, Xi T, Bergé SJ, Maal TJJ (2018) Validation of 3D documentation of palatal soft tissue shape, color, and irregularity with intraoral scanning. Clin Oral Investig 22:1303–1309
Disabato-Mordarski T, Kleinberg I (1996) Measurement and comparison of the residual saliva on various oral mucosal and dentition surfaces in humans. Arch Oral Biol 41:655–665
Farkas LG, Katic MJ, Forrest CR (2007) Comparison of craniofacial measurements of young adult African-American and North American white males and females. Ann Plast Surg 59:692–698
Gracco A, Malaguti A, Lombardo L, Mazzoli A, Raffaeli R (2010) Palatal volume following rapid maxillary expansion in mixed dentition. Angle Orthod 80:153–159
Hallgren KA (2012) Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol 8:23–34
Holmberg KV, Hoffman MP (2014) Anatomy, biogenesis and regeneration of salivary glands. Monogr Oral Sci 24:1–13
Kerr WJ, Kelly J, Geddes DA (1991) The areas of various surfaces in the human mouth from nine years to adulthood. J Dent Res 70:1528–1530
Kho HS (2014) Understanding of xerostomia and strategies for the development of artificial saliva. Chin J Dent Res 17:75–83
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Lee SK, Lee SW, Chung SC, Kim YK, Kho HS (2002) Analysis of residual saliva and minor salivary gland secretions in patients with dry mouth. Arch Oral Biol 47:637–641
Mcgraw K, Wong SP (1996) Forming inferences about some intraclass correlation coefficients. Psychol Methods 1:30–46
Monga N, Kharbanda OP, Balachandran R, Neelapu BC (2020) Palatal volume estimation in operated unilateral and bilateral cleft lip and palate subjects using digital study models. Orthod Craniofac Res 23:284–290
Nascimento WV, Cassiani Rde A, Dantas RO (2013) Effect of gender, height and race on orofacial measurements. Codas 25:149–153
Osailan S, Pramanik R, Shirodaria S, Challacombe SJ, Proctor GB (2011) Investigating the relationship between hyposalivation and mucosal wetness. Oral Dis 17:109–114
Osailan SM, Pramanik R, Shirlaw P, Proctor GB, Challacombe SJ (2012) Clinical assessment of oral dryness: development of a scoring system related to salivary flow and mucosal wetness. Oral Surg Oral Med Oral Pathol Oral Radiol 114:597–603
Oxilia G, Menghi Sartorio JC, Bortolini E, Zampirolo G, Papini A, Boggioni M, Martini S, Marciani F, Arrighi S, Figus C, Marciani G, Romandini M, Silvestrini S, Pedrosi ME, Mori T, Riga A, Kullmer O, Sarig R, Fiorenza L, Giganti M, Sorrentino R, Belcastro MG, Cecchi JM, Benazzi S (2021) Exploring directional and fluctuating asymmetry in the human palate during growth. Am J Phys Anthropol 175:847–864
Pramanik R, Osailan SM, Challacombe SJ, Urquhart D, Proctor GB (2010) Protein and mucin retention on oral mucosal surfaces in dry mouth patients. Eur J Oral Sci 118:245–253
Shahen S, Carrino G, Carrino R, Abdelsalam R, Flores-Mir C, Perillo L (2018) Palatal volume and area assessment on digital casts generated from cone-beam computed tomography scans. Angle Orthod 88:397–402
Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 12:1495–1499
Watanabe S, Dawes C (1990) Salivary flow rates and salivary film thickness in five-year-old children. J Dent Res 69:1150–1153
Wolff M, Kleinberg I (1998) Oral mucosal wetness in hypo- and normosalivators. Arch Oral Biol 43:455–462
Won S, Kho H, Kim Y, Chung S, Lee S (2001) Analysis of residual saliva and minor salivary gland secretions. Arch Oral Biol 46:619–624
Zacharopoulos GV, Manios A, Kau CH, Velagrakis G, Tzanakakis GN, De Bree E (2016) Anthropometric analysis of the face. J Craniofac Surg 27:e71-75
Zhuang Z, Landsittel D, Benson S, Roberge R, Shaffer R (2010) Facial anthropometric differences among gender, ethnicity, and age groups. Ann Occup Hyg 54:391–402
Funding
Z. Assy has received an unrestricted research grant from the Nederlands Tijdschrift voor Tandheelkunde (NTVT) with grant number OZB2018.01.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by Z. Assy. The first draft of the manuscript was written by Z. Assy, and all authors commented on successive versions of the manuscript. All authors read and approved the definitive manuscript.
Corresponding author
Ethics declarations
Competing interests
Z. Assy received an unrestricted research grant from the Nederlands Tijdschrift voor Tandheelkunde (NTVT). D.H.J. Jager declares that he has no conflict of interest, H.S. Brand declares that he has no conflict of interest and F.J. Bikker declares that he has no conflict of interest.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethics Review Committee of the Academic Centre for Dentistry Amsterdam, protocol number 202065) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Availability of data and materials
All data are available upon request from the corresponding author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assy, Z., Jager, D.H.J., Brand, H.S. et al. Correlations of palatal surface area with anthropometric dimensions of the head and face. Surg Radiol Anat 44, 1261–1267 (2022). https://doi.org/10.1007/s00276-022-03008-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-022-03008-4