Abstract
This systematic review was conducted to determine factors that are associated with the degree of hypertrophy of the future liver remnant following portal vein embolization. An extensive search on September 15, 2020, and subsequent literature screening resulted in the inclusion of forty-eight articles with 3368 patients in qualitative analysis, of which 18 studies were included in quantitative synthesis. Meta-analyses based on a limited number of studies showed an increase in hypertrophy response when additional embolization of segment 4 was performed (pooled difference of medians = − 3.47, 95% CI − 5.51 to − 1.43) and the use of N-butyl cyanoacrylate for portal vein embolization induced more hypertrophy than polyvinyl alcohol (pooled standardized mean difference (SMD) = 0.60, 95% CI 0.30 to 0.91). There was no indication of a difference in degree of hypertrophy between patients who received neo-adjuvant chemotherapy and those who did not receive pre-procedural systemic therapy
(pooled SMD = − 0.37, 95% CI − 1.35 to 0.61), or between male and female patients (pooled SMD = 0.19, 95% CI − 0.12 to 0.50).
The study was registered in the International Prospective Register of Systematic Reviews on April 28, 2020 (CRD42020175708).
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Resection of liver tumors plays a central role in the treatment of primary malignancies of the liver and colorectal liver metastases. The most important prerequisite for a safe resection is the presence of an adequate future liver remnant (FLR) that is sufficient to sustain liver function. Postoperative liver failure is still the leading cause of death following major (> 3 segments) liver resection [1].
Several methods to increase the FLR volume and function are available. Hypertrophy of the contralateral liver lobe after vascular obliteration of the hepatic vessels was first identified by James Cantlie in the nineteenth century [2], but the first pre-operative portal vein embolization (PVE) was not performed until 1984 [3]. The success of this minimally invasive procedure, and other more invasive liver augmenting techniques such as Associated Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) to increase the volume and improve the hepatic functional reserve of the FLR tissue prior to resection has allowed for more extensive liver resections and has reduced the risk of postoperative morbidity and mortality [4, 5].
The minimum absolute liver volume necessary to support hepatic function after major liver resection has not been clearly defined. However, a FLR/total liver volume (TLV) ratio of at least 25–30% is recommended in patients with otherwise normal livers and a ratio up to 40% in patients with a compromised liver function [6]. When the FLR/TELV ratio is below these levels, PVE may be performed in an attempt to increase FLR volume.
Identifying factors that predict the degree of hypertrophy of the FLR (i.e., increase in FLR/TLV ratio) after PVE, can improve the selection of patients receiving PVE and more adequately stratify patients as potential surgical candidates. The aim of this systematic review was to find predictive factors for hypertrophy of the FLR after PVE.
Methods
Search Strategy
This review is registered in the International Prospective Register of Systematic Reviews (CRD42020175708). The databases Pubmed/Medline, Embase (ovid) and SCOPUS were searched on July 24, 2019. On September 15, 2020, an update was performed using the same search strategy. The search included the MESH terms “Liver”, “Hypertrophy” and “Embolization, Therapeutic” (Appendix 1).
Inclusion Criteria
Included studies had to meet the following criteria: original research papers, prospective or retrospective studies. The studies had to provide data on factors affecting FLR hypertrophy.
Exclusion Criteria
All case reports and cohort studies reporting results in ten patients or less were excluded; also, in vitro and animal studies, reports concerning surgical approaches and patients with solely arterial embolization were not included. Papers were also excluded when there was overlap with previously published data from the same study group.
Literature Screening
Articles were electronically downloaded into reference management software (Rayyan QCRI and EndNote X7) and duplicated articles were electronically or manually excluded using the Bramer method [7]. Abstracts of the remaining articles were screened by two independent investigators (EAS, BMA) using predefined criteria. Full-text versions of potentially relevant articles were subsequently reviewed by the two investigators and data were extracted. Discrepancy was solved by consensus.
Quality Assessment and Data Extraction
The study quality was assessed by two independent reviewers using the Joana Briggs Institute (JBI) Checklist for Randomized Controlled Trials and Cohort Studies. All information was independently extracted and cross-checked by two investigators according to a standard format as follows: author, publication year, country, study design, population characteristics, PVE segments and technique, FLR volumes pre- and post-PVE, factors affecting FLR hypertrophy, and completion of planned hepatic resection.
Quantitative Synthesis
Standard fixed and random effects model estimates for the meta-analysis of continuous outcomes [8] were calculated for predictive factors for which this was possible and more than two studies could be included. The standardized mean difference (SDM) was calculated, inverse variance weighting was used for pooling and forest plots were constructed. When only median and either range or interquartile range (IQR) were available, mean and standard deviation were estimated [9]. If most studies only reported the sample median and range/IQR of the outcome, the quantile estimation method from McGrath et al. [10] was used. Heterogeneity between studies was assessed using the Q statistic (variation around the average), τ2 (between-study variance), H and I2 (percentage of variation reflecting real differences in effect size). Forest plots displayed I2, τ2 and the p value for the heterogeneity test of Q. If heterogeneity was deemed low (primarily based on a reference cut-off of 25% for I2 and an assessment of τ2), a fixed-effects model was considered appropriate. R package meta was used to perform the analyses (R version 4.0.2).
Results
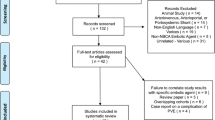
The initial search resulted in 2469 records, of which 120 full-text articles were evaluated and 48 publications were included in qualitative synthesis (Fig. 1). Except for one randomized controlled trial [11], all other included studies had a retrospective study design. The quality of the literature evaluated according to the JBI grades of recommendation showed a ‘Grade A’ in six studies [11,12,13,14,15,16] and ‘Grade B’ in the remaining 42 studies [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58].
The included papers described 3368 patients who underwent pre-operative PVE. The mean age was 62 years (range 17–87). The majority of the patients were men (65.0%). Colorectal liver metastasis (43.8%), cholangiocarcinoma (29.5%), hepatocellular carcinoma (15.2%), and gallbladder carcinoma (2.1%) were the most frequent diagnosis.
PVE approach was mainly transhepatic (81.1%) with n-butyl cyanoacrylate (NBCA (mixture of 1:1–10 with lipiodol), polyvinyl alcohol (PVA, 100–1000 μm) or a combination of these agents with coils or plugs.
2143/3368 (63.6%) patients underwent liver surgery after PVE (Appendix 2). The mean time interval between PVE and liver surgery was 43.2 days (range 23–77). Of 18.7% it was not stated whether subsequent hepatectomy was performed. 17.7% did not undergo surgery, of which 12.9% due to insufficient hypertrophy of the FLR.
Predictive Factors for Hypertrophy of the FLR
Pre- and post-procedural computed tomography (CT) scans were performed to measure the hypertrophy response. In most studies, the absolute volumes were used to calculate the FLR with the formula: (future liver remnant volume (FLRV)/total liver volume (TLV) − tumor volume) × 100%) [12, 13, 15, 16, 18, 21,22,23, 28, 34,35,36,37, 40,41,42, 44, 45, 50, 54, 55, 58]. In other studies, TELV was calculated using CT volumetry and body surface area [14, 19, 20, 25, 29, 31, 38, 39, 53, 56, 57]. The mean time interval between PVE and post-procedural imaging for hypertrophy response was 28.5 days (range 14–56). Identified factors as potential predictors for hypertrophy response of the FLR included embolization-related factors, patient characteristics, quantitative liver function assessment, background liver disease, tumor-related factors and chemotherapy (Table 1). Eighteen studies including 1122 patients were eligible for meta-analysis [14, 16, 18, 20, 22,23,24, 26, 28,29,30,31, 38, 41, 48, 49], which included the factors: “Initial FLR volume”, “Additional embolization of segment 4”, “Embolic agent”, “Chemotherapy” and “Gender”. For each of the remaining factors, only two or less studies reported quantitative information for carrying out a meta-analysis, and this was in consequence not performed.
Initial FLR Volume/Additional Embolization of Segment 4
Thirteen studies, including 1310 patients, stated that the smaller the FLR pre-PVE, the larger the FLR hypertrophy was post-PVE [12, 13, 23, 29, 30, 34, 36, 37, 46, 48, 53,54,55]; this inversely correlated hypertrophy response was confirmed with pooled analyses of three studies that reported correlation coefficients (pooled correlation = − 0.37, 95%CI − 0.65 to 0.00, Fig. 2), though with a high degree of heterogeneity (I2 = 92%, τ2 = 0.1043, p < 0.01).
When a trisectionectomy was planned, right-PVE (RPVE) with additional embolization of segment 4 (S4) was generally performed. Six studies, including a total of 365 patients, reported the effect of additional S4 embolization on the degree of hypertrophy. Three studies, with a total of 207 patients, found a significant increase in FLR hypertrophy with additional embolization of S4 [14, 20, 29]. Whereas three other studies with a total of 153 patients found no significant difference between RPVE with or without the addition of S4 [23, 38, 57]. Four studies were eligible for meta-analysis; three of these studies displayed only medians and range of degree of hypertrophy and transformations were needed to impute mean and standard deviation and obtain the standardized mean difference. It was assumed that for one study reporting only mean and standard deviation, the degree of hypertrophy distribution was assumed to be normal, and thus sample medians were estimated by the sample means and their variances were estimated by the sample variances divided by the number of subjects. When the medians and ranges were employed in the quantile estimation method [10], a difference in favor of RPVE + S4 was found (pooled difference of medians = − 3.47, 95% CI − 5.51 to − 1.43, Fig. 3).
Embolic Agent
The embolization materials mainly used were NBCA and PVA with or without coils or plugs. Three studies showed a higher hypertrophy response in 94/196 (48.0%) patients treated with NBCA ± Amplatzer-plug compared to patients treated with PVA ± coils [26, 28, 31]. The mean differences of quantitative analysis indicate that there is a significant difference of degree of hypertrophy in favor of NBCA (pooled SMD = 0.60, 95% CI 0.30 to 0.91, Fig. 4).
Chemotherapy
Chemotherapy has potential negative side effects in the liver, most notable a non-tumoral liver parenchymal injury known as sinusoidal obstruction syndrome (SOS). There is a higher incidence of SOS in patients who received extensive (≥ 6 cycles) oxaliplatin-based chemotherapy regimens [15]. A lower hypertrophy response was seen in patients suffering from SOS (11/42, 26.2%) with an increase in the FLR of 16.8%, compared to an FLR increase of 55.6% in patients without SOS) [15]. However, in the same study, 64.3% of the patients received oxaliplatin-based neo-adjuvant chemotherapy, which showed similar hypertrophy response compared to non-oxaliplatin-based neo-adjuvant chemotherapy regimens.
Regarding neo-adjuvant chemotherapy in general there are only three cohort studies that published a significant negative influence of chemotherapy on hypertrophy response [18, 34, 52]. Many other studies, including larger cohorts, could not support this finding (Table 1) [16, 22,23,24, 26, 27, 35, 39, 41, 46, 48, 49, 54, 57]. Pooled data showed no indication of a difference in degree of hypertrophy between patients receiving neo-adjuvant chemotherapy compared to patients who did not receive pre-procedural systemic treatment (Fig. 5). There is, however, a very high degree of heterogeneity in this relatively low number of studies (I2 = 92%, τ2 = 48.48, p < 0.01).
Gender
Gender was not associated with hypertrophy response in sixteen studies including 1647 patients (Table 1), which was also not significant after pooling data of three studies [23, 42, 44] with a SMD = 0.19, 95% CI − 0.12 to 0.50 (I2 = 0, τ2 = 0, p = 0.85, Fig. 6).
Discussion
Although a wide range of pre-procedural factors was evaluated in the current review, only a few factors were eligible for meta-analyses, and for each of them, only a small number of studies contained the quantitative information needed for performing a meta-analysis. The included studies showed an inversely correlated hypertrophy response after PVE to the pre-embolization FLR volume: the smaller the FLR pre-PVE, the larger the FLR hypertrophy was post-PVE. Meta-analyses showed indications that the degree of hypertrophy was higher in patients with additional embolization of S4, compared to patients in whom only the right liver was embolized. Liver regeneration is a complex process, and the exact pathophysiological situation following PVE remains unclear. It is known that various cytokines, growth factors, vasoregulators, hormones and proteins initiate hepatocyte proliferation [4, 59]. It could be considered that the FLR volume will increase more if the embolized area is more extensive.
Different embolic agents have been used for PVE. NBCA and PVA, or a combination of these agents with coils/plugs is the mainly used non-absorbable materials, which lead to persistent occlusion of the portal branches, preventing peripheral recanalization. Pooled data showed a statistically significant higher degree of hypertrophy after embolization with NBCA compared to PVA ± coils. Superior increase in liver volume with NBCA plus iodized oil versus PVA plus coils was also reported in a recently published randomized controlled trial by Luz et al. [60].
Chemotherapy by means of downstaging allows patients with initially unresectable liver tumors to become resectable, which has led to an increase in exposure to chemotherapy in induction setting [61]. Previous reports showed the influence of the duration and the type of the neo-adjuvant chemotherapy on the postoperative morbidity and mortality after major hepatectomy [62, 63]; this suggests that the duration and type of chemotherapy would also affect liver regeneration after PVE. In a study by Narita et al. [15] SOS caused by oxaliplatin-based chemotherapy, inhibited FRL hypertrophy after PVE and induced postoperative liver failure. However, pooled data showed that there is no significant difference in degree of hypertrophy between patients receiving neo-adjuvant chemotherapy, including oxaliplatin-based agents, compared to patients who did not receive pre-procedural chemotherapy.
Although conventional PVE has been standard of care for the past two decades, newer approaches have been used in an attempt to increase liver hypertrophy. In patients with HCC, TACE has an anti-tumoral effect and may help to occlude arterio-portal shunts; these shunts are thought to negatively impact FLR growth [64]. Significant increase in FLR volume has been described in patients who underwent sequential TACE and PVE compared to PVE alone [50, 65], which was also noticed in a systematic review including four publications and 171 patients [66].
Novel promising liver augmenting techniques are being investigated, such as liver venous deprivation (LVD) [67]. This is a procedure in which not only the portal vein, but also the hepatic vein is embolized. The hepatic outflow obstruction induces hemodynamic changes with decrease in hepatic arterial inflow, which causes more damage to the embolized lobe [68]. This promising technique was only described in a few original research papers with limited patient numbers; therefore, these studies were not included in the current review. Guiu et al. [67] were the first to describe this technique with good results in a series of seven patients. Larger prospective trails are under way to define the role of LVD to increase the FLR.
Applications of techniques to enhance liver regeneration rely on an adequate assessment of the regenerative response of the FLR. Imaging-based volumetry is the golden standard in order to determine whether the hypertrophy response of the FLR is sufficient and safe resection can be undertaken [69]. However, volumetric assessment does not provide quantitative information of the liver. Newer imaging techniques to assess the functional share of the FLR are emerging. With HBS in combination with SPECT-CT functional and anatomic information are combined to assess segmental liver function. Using this nuclear imaging technique after PVE, showed that the functional response exceeded the volumetric response, suggesting that the waiting time to resection can be decreased [16, 70]. Functional imaging with Magnetic Resonance Imaging (MRI) with gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid can also be used for the assessment regional liver function, with the advantage of characterization of liver lesions and the assessment of parenchymal disease [71, 72]. However, the assessment of liver function with MRI is still under investigation.
Radiomics uses a high throughput extraction of large amounts of quantitative imaging features with the intent of creating mineable databases from radiological images [73]. This advanced image analysis and mining of conventional medical imaging is able to capture additional information not currently used. Two previous studies showed that quantitative imaging features of the liver parenchyma correlated with hepatic insufficiency after major hepatic resection [74] and the rate of liver regeneration after liver transplantation [75]. As yet, radiomics has not been used to predict the liver hypertrophy after PVE. This innovation in medical imaging analysis might provide for biomarkers, which can be used to improve the patient selection for liver enhancing technique.
The primary limitation of this systematic review is the quality of the available literature. Most of the included articles showed a ‘Grade B’ quality according to the JBI quality assessment tool and had a retrospective design with small sample size. Due to this limited quality and the observational nature of the data, potential confounding factors could bias results. In addition, between-study heterogeneity could be influenced by the differences in inclusion criteria such as patient population, PVE technique and volumetry measurement. Besides, not all studies report which formula or method was used to measure the hypertrophy ratio. Finally, it is not clear what the criterion is in the different papers for reporting either mean and standard deviation, or median, sample size or range/IQR. For obtaining the pooled SMD with the inverse variance approach, studies reporting sample medians should either be excluded from the synthesis, or mean and standard deviation should be estimated using a transformation-based method. Applying these transformations when the data are skewed might produce biased results. This, together with the fact that only a small number of studies could be used for each factor, means that results from meta-analyses should be taken with caution.
Conclusion
The degree of hypertrophy was found to be more pronounced when NBCA was used as embolic agent and when a larger volume was embolized. Neo-adjuvant chemotherapy and gender do not influence the degree of hypertrophy response. Due to the quality level and heterogeneity of the included studies and lack of randomized controlled trials, no other conclusions could be drawn. Techniques that may improve patient selection for a liver regenerating procedure and more adequately stratify patients as surgical candidates remain a subject of further research.
References
Ribeiro HS, et al. Extended preoperative chemotherapy, extent of liver resection and blood transfusion are predictive factors of liver failure following resection of colorectal liver metastasis. Eur J Surg Oncol. 2013;39(4):380–5.
Cantlie J. On a new arrangement of the right and left lobes of the liver. J Anat Physiol. 1898;32:4–10.
Makuuchi M, et al. Preoperative transcatheter embolization of the portal venous branch for patients receiving extended lobectomy due to the bile duct carcinoma. J Jpn Pract Surg Soc. 1984;45(12):1558–64.
Clavien PA, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356(15):1545–59.
Schadde E, et al. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol. 2015;22(9):3109–20.
Loffroy R, et al. Preoperative portal vein embolization in liver cancer: indications, techniques and outcomes. Quant Imaging Med Surg. 2015;5(5):730–9.
Bramer WM, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–3.
Borenstein M, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111.
Wan X, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
McGrath S, et al. Meta-analysis of the difference of medians. Biometr J. 2020;62(1):69–98.
Beppu T, et al. Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: a randomized controlled trial. J Gastroenterol. 2015;50(12):1197–205.
Denys A, et al. Portal vein embolization with N-butyl cyanoacrylate before partial hepatectomy in patients with hepatocellular carcinoma and underlying cirrhosis or advanced fibrosis. J Vasc Interv Radiol. 2005;16(12):1667–74.
Hocquelet A, et al. Point-shear wave elastography predicts liver hypertrophy after portal vein embolization and postoperative liver failure. Diagn Interv Imaging. 2018;99(6):371–9.
Ito J, et al. Evaluation of segment 4 portal vein embolization added to right portal vein for right hepatic trisectionectomy: a retrospective propensity score-matched study. J Hepatobiliary Pancreat Sci. 2020;27(6):299–306.
Narita M, et al. Sinusoidal obstruction syndrome compromises liver regeneration in patients undergoing two-stage hepatectomy with portal vein embolization. Surg Today. 2011;41(1):7–17.
Rassam F, et al. Functional and volumetric assessment of liver segments after portal vein embolization: differences in hypertrophy response. Surgery. 2018;165(4):686–95.
am Esch JS, et al. Infusion of CD133+ bone marrow-derived stem cells after selective portal vein embolization enhances functional hepatic reserves after extended right hepatectomy: a retrospective single-center study. Ann Surg. 2012;255(1):79–85.
Beal IK, et al. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. Br J Radiol. 2006;79(942):473–8.
Biggemann L, et al. Future liver remnant growth after various portal vein embolization regimens: a quantitative comparison. Minim Invasive Ther Allied Technol. 2020;29(2):98–106.
Bjornsson B, et al. Segment 4 occlusion in portal vein embolization increase future liver remnant hypertrophy—a Scandinavian cohort study. Int J Surg. 2020;75:60–5.
Capussotti L, et al. Extension of right portal vein embolization to segment IV portal branches. Arch Surg. 2005;140(11):1100–3.
Covey AM, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247(3):451–5.
de Baere T, et al. Predictive factors for hypertrophy of the future remnant liver after selective portal vein embolization. Ann Surg Oncol. 2010;17(8):2081–9.
Deipolyi AR, et al. Portal vein embolization: impact of chemotherapy and genetic mutations. J Clin Med. 2017;6(3):26.
Denbo JW, et al. Overall body composition and sarcopenia are associated with poor liver hypertrophy following portal vein embolization. J Gastrointest Surg. 2020;25(2):405–10.
Dhaliwal SK, et al. Portal vein embolization: correlation of future liver remnant hypertrophy to type of embolic agent used. Can Assoc Radiol J. 2018;69(3):316–21.
Goéré D, et al. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J Gastrointest Surg. 2006;10(3):365–70.
Guiu B, et al. Portal vein embolization before right hepatectomy: improved results using n-butyl-cyanoacrylate compared to microparticles plus coils. Cardiovasc Intervent Radiol. 2013;36(5):1306–12.
Hammond CJ, et al. Segment 2/3 hypertrophy is greater when right portal vein embolisation is extended to segment 4 in patients with colorectal liver metastases: a retrospective cohort study. Cardiovasc Intervent Radiol. 2019;42(4):552–9.
Igami T, et al. Portal vein embolization using absolute ethanol: evaluation of its safety and efficacy. J Hepatobiliary Pancreat Sci. 2014;21(9):676–81.
Jaberi A, et al. Comparison of clinical outcomes following glue versus polyvinyl alcohol portal vein embolization for hypertrophy of the future liver remnant prior to right hepatectomy. J Vasc Interv Radiol. 2016;27(12):1897-1905.e1.
Kaido T, et al. Portal embolization in various types of liver: novel variables to predict hypertrophy. Hepatogastroenterology. 2003;50(49):140–5.
Kaneko T, Nakao A, Takagi H. Clinical studies of new material for portal vein embolization: comparison of embolic effect with different agents. Hepatogastroenterology. 2002;49(44):472–7.
Kasai Y, et al. Prediction of the remnant liver hypertrophy ratio after preoperative portal vein embolization. Eur Surg Res. 2013;51(3–4):129–37.
Kohno S, et al. Portal vein embolization: radiological findings predicting future liver remnant hypertrophy. AJR Am J Roentgenol. 2020;214(3):687–93.
Luz JHM, et al. Portal vein embolization with n-butyl-cyanoacrylate through an ipsilateral approach before major hepatectomy: single center analysis of 50 consecutive patients. Cancer Imaging. 2017;17(1):25.
Malinowski M, et al. Factors influencing hypertrophy of the left lateral liver lobe after portal vein embolization. Langenbecks Arch Surg. 2015;400(2):237–46.
Massimino KP, et al. Safety and efficacy of preoperative right portal vein embolization in patients at risk for postoperative liver failure following major right hepatectomy. HPB (Oxford). 2012;14(1):14–9.
Mise Y, et al. A nomogram to predict hypertrophy of liver segments 2 and 3 after right portal vein embolization. J Gastrointest Surg. 2016;20(7):1317–23.
Miura S, et al. Preoperative biliary drainage of the hepatic lobe to be resected does not affect liver hypertrophy after percutaneous transhepatic portal vein embolization. Surg Endosc. 2020;34(2):667–74.
Nafidi O, et al. Hypertrophy of the non-embolized liver after chemotherapy. HPB (Oxford). 2009;11(2):103–7.
Nanashima A, et al. Relationship of hepatic functional parameters with changes of functional liver volume using technetium-99m galactosyl serum albumin scintigraphy in patients undergoing preoperative portal vein embolization: a follow-up report. J Surg Res. 2010;164(2):e235–42.
Peng PD, et al. Sequential intra-arterial therapy and portal vein embolization is feasible and safe in patients with advanced hepatic malignancies. HPB (Oxford). 2012;14(8):523–31.
Sakakibara M, et al. Preoperative portal vein embolization before major hepatectomy in patients with excess bilirubin does not affect hypertrophy of remnant liver and postoperative outcomes. Hepatogastroenterology. 2014;61(132):908–15.
Schulze-Hagen M et al. Correlation between sarcopenia and growth rate of the future liver remnant after portal vein embolization in patients with colorectal liver metastases. Cardiovasc Intervent Radiol. 2020.
Simoneau E, et al. Neoadjuvant chemotherapy does not impair liver regeneration following hepatectomy or portal vein embolization for colorectal cancer liver metastases. J Surg Oncol. 2016;113(4):449–55.
Sun JH, et al. Effects of liver cirrhosis on portal vein embolization prior to right hepatectomy in patients with primary liver cancer. Oncol Lett. 2018;15(2):1411–6.
Takahashi EA, Fleming CJ, Andrews JC. Future liver remnant hypertrophy after portal vein embolization is inversely correlated with intrahepatic tumor burden. J Vasc Interv Radiol. 2019;30(3):435–9.
Tanaka K, et al. Influence of chemotherapy on liver regeneration induced by portal vein embolization or first hepatectomy of a staged procedure for colorectal liver metastases. J Gastrointest Surg. 2010;14(2):359–68.
Terasawa M, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization alone before major hepatectomy for patients with large hepatocellular carcinoma: an intent-to-treat analysis. Surgery. 2020;167(2):425–31.
Treska V, et al. Portal vein embolization (PVE) versus PVE with haematopoietic stem cell application in patients with primarily non-resectable colorectal liver metastases. Anticancer Res. 2018;38(9):5531–7.
Treska V, et al. Prognostic importance of some clinical and therapeutic factors for the effect of portal vein embolization in patients with primarily inoperable colorectal liver metastases. Arch Med Sci. 2013;9(1):47–54.
Wakabayashi H, et al. Application of preoperative portal vein embolization before major hepatic resection in patients with normal or abnormal liver parenchyma. Surgery. 2002;131(1):26–33.
Watanabe N, et al. A predictive scoring system for insufficient liver hypertrophy after preoperative portal vein embolization. Surgery. 2018;163(5):1014–9.
Yamashita S, et al. Efficacy of preoperative portal vein embolization among patients with hepatocellular carcinoma, biliary tract cancer, and colorectal liver metastases: a comparative study based on single-center experience of 319 cases. Ann Surg Oncol. 2017;24(6):1557–68.
Yim J, et al. Effect of hyperbilirubinemia on hepatic hypertrophy after portal vein embolization and liver failure after hepatectomy in primary biliary malignancy. J Vasc Interv Radiol. 2019;30(1):31–7.
Zeile M, et al. Identification of cofactors influencing hypertrophy of the future liver remnant after portal vein embolization-the effect of collaterals on embolized liver volume. Br J Radiol. 2016;89(1068):20160306.
Farges O, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237(2):208–17.
Le Roy B, et al. Liver hypertrophy: underlying mechanisms and promoting procedures before major hepatectomy. J Visc Surg. 2018;155(5):393–401.
Luz JHM et al. BestFLR trial: liver regeneration at CT before major hepatectomies for liver cancer—a randomized controlled trial comparing portal vein embolization with n-butyl-cyanoacrylate plus iodized oil versus polyvinyl alcohol particles plus coils. Radiology. 204055.
Adam R, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–57 (discussion 657-8).
Karoui M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243(1):1–7.
Vauthey JN, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24(13):2065–72.
Pawlik TM, et al. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11(7):860–8.
Yoo H, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18(5):1251–7.
Piardi T, et al. Management of large hepatocellular carcinoma by sequential transarterial chemoembolization and portal vein embolization: a systematic review of the literature. Minerva Chir. 2016;71(3):192–200.
Guiu B, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016;26(12):4259–67.
Ko GY, et al. Interventional oncology: new options for interstitial treatments and intravascular approaches: right hepatic vein embolization after right portal vein embolization for inducing hypertrophy of the future liver remnant. J Hepatobiliary Pancreat Sci. 2010;17(4):410–2.
Shoup M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7(3):325–30.
Rassam F, et al. Comparison of functional and volumetric increase of the future remnant liver and postoperative outcomes after portal vein embolization and complete or partial associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Ann Transl Med. 2020;8(7):436.
Rassam F, et al. Comparison between dynamic gadoxetate-enhanced MRI and (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for quantitative assessment of liver function. Eur Radiol. 2019;29(9):5063–72.
Ryeom HK, et al. Quantitative evaluation of liver function with MRI using Gd-EOB-DTPA. Korean J Radiol. 2004;5(4):231–9.
Kumar V, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30(9):1234–48.
Simpson AL, et al. Texture analysis of preoperative CT images for prediction of postoperative hepatic insufficiency: a preliminary study. J Am Coll Surg. 2015;220(3):339–46.
Kim JE, et al. Prediction of liver remnant regeneration after living donor liver transplantation using preoperative CT texture analysis. Abdom Radiol (NY). 2019;44(5):1785–94.
Acknowledgements
Not applicable
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent for publication is not requited.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soykan, E.A., Aarts, B.M., Lopez-Yurda, M. et al. Predictive Factors for Hypertrophy of the Future Liver Remnant After Portal Vein Embolization: A Systematic Review. Cardiovasc Intervent Radiol 44, 1355–1366 (2021). https://doi.org/10.1007/s00270-021-02877-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-02877-3