Abstract
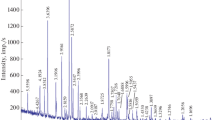
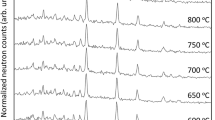
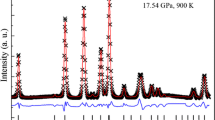
The heat capacity (C p ) of dmitryivanovite synthesized with a cubic press was measured in the temperature range of 5–664 K using the heat capacity option of a physical properties measurement system and a differential scanning calorimeter. The entropy of dmitryivanovite at standard temperature and pressure (STP) was calculated to be 110.1 ± 1.6 J mol−1 K−1 from the measured C p data. With the help of new phase equilibrium experiments done at 1.5 GPa, the phase transition boundary between krotite and dmitryivanovite was best represented by the equation: P (GPa) = −2.1825 + 0.0025 T (K). From the temperature intercept of this phase boundary and other available thermodynamic data for krotite and dmitryivanovite, the enthalpy of formation and Gibbs free energy of formation of dmitryivanovite at STP were calculated to be −2326.7 ± 2.1 and −2,208.1 ± 2.1 kJ mol−1, respectively. It is also inferred that dmitryivanovite is the stable CaAl2O4 phase at STP and has a wide stability field at high pressures whereas the stability field of krotite is located at high temperatures and relatively low pressures. This conclusion is consistent with natural occurrences (in Ca–Al-rich inclusions) of dmitryivanovite and krotite, where the former is interpreted as the shock metamorphic product of originally present krotite.



Similar content being viewed by others
References
Akaogi M (1999) High pressure transitions in the system MgAl2O4–CaAl2O4: a new hexagonal aluminous phase with implication for the lower mantle. Phys Earth Planet Inter 115(1):67–77
Akaogi M, Takayama H, Kojitani H, Kawaji H, Atake T (2007) Low-temperature heat capacities, entropies and enthalpies of Mg2SiO4 polymorphs, and α-β-γ and post-spinel phase relations at high pressure. Phys Chem Miner 34(3):169–183. doi:10.1007/s00269-006-0137-3
Akaogi M, Kojitani H, Morita T, Kawaji H, Atake T (2008) Low-temperature heat capacities, entropies and high-pressure phase relations of MgSiO3 ilmenite and perovskite. Phys Chem Miner 35(5):287–297. doi:10.1007/s00269-008-0222-x
Allen JM, Grossman L, Davis AM, Hutcheon ID (1978) Mineralogy, textures, and mode of formation of a hibonite-bearing Allende inclusion. In: Proc Lunar Planet Sci Conf 9th, pp 1209–1233
Allibert M, Chatillon C, Jacob K, Lourtau R (1981) Mass spectrometric and electrochemical studies of thermodynamic properties of liquid and solid phases in the system CaO–Al2O3. J Am Ceram Soc 64(5):307–314
Armstrong JT, Meeker GP, Huneke JC, Wasserburg GJ (1982) The Blue Angel: I. The mineralogy and petrogenesis of a hibonite inclusion from the Murchison meteorite. Geochim Cosmochim Acta 46(4):575–595. doi:10.1016/0016-7037(82)90160-0
Berman RG, Brown TH (1985) Heat capacity of minerals in the system Na2O–K2O–CaO–MgO–FeO–Fe2O3–Al2O3–SiO2–TiO2–H2O–CO2: representation, estimation, and high temperature extrapolation. Contr Miner Petrol 89(2):168–183
Boerio-Goates J, Stevens R, Hom BK, Woodfield BF, Piccione PM, Davis ME, Navrotsky A (2002) Heat capacities, third-law entropies and thermodynamic functions of SiO2 molecular sieves from T = 0 K to 400 K. J Chem Thermodyn 34(2):205–227. doi:10.1006/jcht.2001.0900
Bonnickson KR (1955) High temperature heat contents of aluminates of calcium and magnesium. J Phys Chem 59(3):220–221
Bouvier A, Wadhwa M (2010) The age of the Solar System redefined by the oldest Pb–Pb age of a meteoritic inclusion. Nat Geosci 3(9):637–641
Chou CL, Baedecker PA, Wasson JT (1976) Allende inclusions: volatile-element distribution and evidence for incomplete volatilization of presolar solids. Geochim Cosmochim Acta 40:85–94
Christophe Michel-Lévy M, Kurat G, Brandstätter F (1982) A new calcium-aluminate from a refractory inclusion in the Leoville carbonaceous chondrite. Earth Planet Sci Lett 61(1):13–22. doi:10.1016/0012-821x(82)90033-4
Coughlin JP (1956) Heats of Formation of Crystalline CaO·Al2O3, 12CaO·7Al2O3, and 3CaO·Al2O3. J Am Chem Soc 78(21):5479–5482
Dachs E, Benisek A (2011) A sample saving method for heat capacity measurements on powders using relaxation calorimetry. Cryogenics 51:460–464. doi:10.1016/j.cryogenics.2011.04.011
Dachs E, Bertoldi C (2005) Precision and accuracy of the heat-pulse calorimetric technique: low-temperature heat capacities of milligram-sized synthetic mineral samples. Eur J Miner 17(2):251–259. doi:10.1127/0935-1221/2005/0017-0251
Dachs E, Harlov D, Benisek A (2010) Excess heat capacity and entropy of mixing along the chlorapatite–fluorapatite binary join. Phys Chem Miner 37:1–12
Ditmars DA, Ishihara S, Chang SS, Bernstein G, West ED (1982) Measurements of the relative enthalpy of pure α-Al2O3 (NBS heat capacity and enthalpy standard reference material no. 720) from 10 to 1,950 K. J Res Nat Bur Stand 87:5–9
Ebel DS (2006) Condensation of rocky material in astrophysical environments. Meteorit Early Solar Syst II 1:253–277
Ebel DS, Grossman L (2000) Condensation in dust-enriched systems. Geochim Cosmochim Acta 64(2):339–366
Eliezer N, Howald RA (1982) Reply to Hemingway’s comment on” Thermodynamic properties of calcium aluminates”. J Phys Chem 86(14):2803–2804
Eliezer I, Eliezer N, Howald RA, Viswanadham P (1981) Thermodynamic properties of calcium aluminates. J Phys Chem 85(19):2835–2838. doi:10.1021/j150619a028
Fuchs LH, Olsen E, Jensen KJ (1973) Mineralogy, mineral-chemistry, and composition of the Murchison (C2) meteorite. Smithsonian Contributions to the earth sciences, no. 10. pp 1–39
Geiger C, Kleppa O, Mysen B, Lattimer J, Grossman L (1988) Enthalpies of formation of CaAl4O7 and CaAl12O19 (hibonite) by high temperature, alkali borate solution calorimetry. Geochim Cosmochim Acta 52(6):1729–1736
Grossman L (1972) Condensation in the primitive solar nebula. Geochim Cosmochim Acta 36(5):597–619
Grossman L (1975) Petrography and mineral chemistry of ca-rich inclusions in allende meteorite. Geochim Cosmochim Acta 39(4):433–454
Grossman L (1980) Refractory inclusions in the Allende meteorite. Annu Rev Earth Pl Sc 8:559–608
Grossman L, Ebel DS, Simon SB, Davis AM, Richter FM, Parsad NM (2000) Major element chemical and isotopic compositions of refractory inclusions in C3 chondrites: the separate roles of condensation and evaporation. Geochim Cosmochim Acta 64(16):2879–2894
Grossman L, Simon S, Rai V, Thiemens M, Hutcheon I, Williams R, Galy A, Ding T, Fedkin A, Clayton R (2008) Primordial compositions of refractory inclusions. Geochim Cosmochim Acta 72(12):3001–3021. doi:10.1016/j.gca.2008.04.002
Hemingway BS (1982) Comment on “Thermodynamic properties of calcium aluminates”. J Phys Chem 86(14):2802–2803
Hofmeister AM (2004) Physical properties of calcium aluminates from vibrational spectroscopy. Geochim Cosmochim Acta 68(22):4721–4726
Hofmeister AM, Wopenka B, Locock AJ (2004) Spectroscopy and structure of hibonite, grossite, and CaAl2O4: Implications for astronomical environments. Geochim Cosmochim Acta 68(21):4485–4503. doi:10.1016/j.gca.2004.03.011
Ito S, Suzuki K, Inagaki M (1980) High-pressure modifications of CaAl2O4 and CaGa2O4. Mater Res Bull 15(c):925–932
Ivanova MA, Petaev MI, Macpherson GJ, Nazarov MA, Taylor LA, Wood JA (2002) The first known natural occurrence of calcium monoaluminate, in a calcium-aluminum-rich inclusion from the CH chondrite Northwest Africa 470. Meteorit Planet Sci 37(10):1337–1344
Keil K, Fuchs LH (1971) Hibonite Ca2(Al, Ti)24O38 from leoville and allende chondritic meteorites. Earth Planet Sci Lett 12(2):184–190
Kennedy C, Stancescu M, Marriott R, White M (2007) Recommendations for accurate heat capacity measurements using a Quantum Design physical property measurement system. Cryogenics 47(2):107–112. doi:10.1016/j.cryogenics.2006.10.001
King EG (1955) Heat Capacities at Low Temperatures and Entropies at 298.16 K. of Crystalline Calcium and Magnesium Aluminates. J Phys Chem 59(3):218–219
Klemme S, O’Neill HSC, Schnelle W, Gmelin E (2000) The heat capacity of MgCr2O4, FeCr2O4, and Cr2O3 at low temperatures and derived thermodynamic properties. Am Miner 85:1686–1693
Koehler MF, Barany R, Kelley KK (1961) Heats and free energies of formation of ferrites and aluminates of calcium, magnesium, sodium, and lithium. Bureau of Mines, Berkeley
Krot AN, McKeegan KD, Russell SS, Meibom A, Weisberg MK, Zipfel J, Krot TV, Fagan TJ, Keil K (2001) Refractory calcium-aluminum-rich inclusions and aluminum-diopside-rich chondrules in the metal-rich chondrites Hammadah al Hamra 237 and Queen Alexandra Range 94411. Meteorit Planet Sci 36(9):1189–1216
Kumar RV, Kay DAR (1985) The utilization of galvanic cells using Caβ’’-Alumina solid electrolytes in a thermodynamic investigation of the CaO-Al2O3 system. Metall Trans B 16(1):107–112
Kuzmenko V, Uspenskaya I, Rudnyi E (1997) Simultaneous assessment of thermodynamic functions of calcium aluminates. Bull Soc Chim Belg 106(5):235–244
Lashley JC, Hundley MF, Migliori A, Sarrao JL, Pagliuso PG, Darling TW, Jaime M, Cooley JC, Hults WL, Morales L (2003) Critical examination of heat capacity measurements made on a Quantum Design physical property measurement system. Cryogenics 43(6):369–378
Lazic B, Kahlenberg V, Konzett J (2007) Structural studies on a stuffed framework high pressure polymorph of CaAl2O4. Z Kristallogr 222(12/2007):690–695
Ma C, Kampf AR, Connolly HC Jr, Beckett JR, Rossman GR, Smith SAS, Schrader DL (2011) Krotite, CaAl2O4, a new refractory mineral from the NWA 1934 meteorite. Am Miner 96(5–6):709–715. doi:10.2138/am.2011.3693
Maruyama S, Tomioka N (2011) Ca-Al-Fe rich inclusion in the Vigarano CV3 chondrite. Meteorit Planet Sci 46(5):690–700
Mikouchi T, Zolensky M, Ivanova M, Tachikawa O, Komatsu M, Le L, Gounelle M (2009) Dmitryivanovite: A new high-pressure calcium aluminum oxide from the Northwest Africa 470 CH3 chondrite characterized using electron backscatter diffraction analysis. Am Miner 94(5–6):746–750. doi:10.2138/am.2009.3080
Mraw SC (1988) Differential scanning calorimetry. In: Ho CY (ed) Specific Heat of Solids, CINDAS Data Series on Material Properties, vol 1–2. Hemisphere Publishing Corporation, New York, pp 395–437
Navrotsky A, Peraudeau G, McMillan P, Coutures JP (1982) A thermochemical study of glasses and crystals along the joins silica-calcium aluminate and silica-sodium aluminate. Geochim Cosmochim Acta 46(11):2039–2047
Notsu K, Onuma N, Nishida N, Nagasawa H (1978) High temperature heating of the Allende meteorite. Geochim Cosmochim Acta 42(6):903–907. doi:10.1016/0016-7037(78)90101-1
Popov SG, Levitskii VA, Skolis YY, Karlin VV (1979) Thermodynamic stability of calcium hexaaluminate CaO·6Al2O3 and its reactions with the oxides of Ti Cr and Zr. Inorg Mater 15(7):968–971
Reid AF, Ringwood AE (1969) Newly observed high pressure transformations in Mn3O4, CaAl2O4, and ZrSiO4. Earth Planet Sci Lett 6(3):205–208. doi:10.1016/0012-821x(69)90091-0
Robie RA, Hemingway BS (1972) Calorimeters for heat of solution and low-temperature heat capacity measurements. US Gov. Print. Off, Washington
Robie RA, Hemingway BS (1995) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. US Geol Survey Bull 2131:461
Scott ERD, Keil K, Stoffler D (1992) Shock metamorphism of carbonaceous chondrites. Geochim Cosmochim Acta 56(12):4281–4293
Secco RA (1994) Load cycling and pressure efficiency in a large volume press. In: Schmidt SC, Shaner JW, Samara GA, Ross M (eds) High pressure science and technology, AIP Proc. #309, vol 2, New York, pp 1593–1596
Simon S, Yoneda S, Grossman L, Davis A (1994) A CaAl4O7-bearing refractory spherule from Murchison: Evidence for very high-temperature melting in the solar nebula. Geochim Cosmochim Acta 58(8):1937–1949
Skolis YY, Levitskii VA, Yanishevskii VM (1981) Thermodynamics of double oxides - thermodynamic properties of CaAl4O7 and CaAl12O19 at high-temperatures. Zh Fiz Khim 55(1):46–50
Sweeney Smith S, Connolly H, Ma C, Rossman G, Beckett J, Ebel D, Schrader D (2010) Initial Analysis of a Refractory Inclusion Rich in CaAl2O4 from NWA 1934: Cracked Egg. 41st Lunar and Planetary Science Conference, p 1877
Weber D, Bischoff A (1994) The occurrence of grossite (CaAl4O7) in chondrites. Geochim Cosmochim Acta 58(18):3855–3877
Weisberg MK, Prinz M, Clayton RN, Mayeda TK, Grady MM, Pillinger CT (1995) The CR chondrite clan. Proc NIPR Symp Antarct Meteorit 8:11–32
Westrich HR, Navrotsky A (1981) Some thermodynamic properties of fluorapatite, fluorpargasite and fluorphlogopite. Am J Sci 281(8):1091–1103
Yong W, Dachs E, Withers AC, Essene EJ (2006) Heat capacity and phase equilibria of hollandite polymorph of KAlSi3O8. Phys Chem Miner 33(3):167–177. doi:10.1007/s00269-006-0063-4
Yong W, Dachs E, Withers AC, Essene EJ (2007) Heat capacity of γ-Fe2SiO4 between 5 and 303 K and derived thermodynamic properties. Phys Chem Miner 34(2):121–127. doi:10.1007/s00269-006-0133-7
Yong W, Dachs E, Withers AC, Essene EJ (2008) Heat capacity and phase equilibria of wadeite-type K2 Si4O9. Contr Miner Petrol 155(2):137–146. doi:10.1007/s00410-007-0232-6
Acknowledgments
The authors gratefully acknowledge the constructive reviews of H Long, which improved the quality of the manuscript. This work was supported by an NSERC Discovery Grant to R. A. Secco, NASA grant NNX11AG64G to A. C. Withers, and grants P21370-N21 and P23056-N21 of the Austrian Science Fund (FWF) to E. Dachs and A. Benisek.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, W., Dachs, E., Benisek, A. et al. Heat capacity, entropy, and phase equilibria of dmitryivanovite. Phys Chem Minerals 39, 259–267 (2012). https://doi.org/10.1007/s00269-012-0481-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-012-0481-4