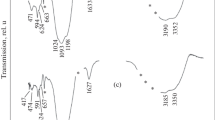
Abstract—
A thermochemical study of natural calcium and magnesium orthosilicate monticellite (Ca1.00Mg0.95\({\text{Fe}}_{{0.05}}^{{2 + }}\))[SiO4] from the Khabarovsk Territory, Russia, was carried out on a Tian–Calvet microcalorimeter. The enthalpy of formation from elements Δf\(H_{{{\text{el}}}}^{^\circ }\)(298.15 K) = –2238.4 ± 4.5 kJ/mol was determined by high-temperature melt solution calorimetry. The enthalpy and Gibbs energy of formation of monticellite of theoretical composition of CaMg[SiO4] are calculated: Δf\(H_{{{\text{el}}}}^{^\circ }\)(298.15 K) = –2248.4 ± 4.5 kJ/mol and Δf\(G_{{{\text{el}}}}^{^\circ }\)(298.15 K) = –2130.5 ± 4.5 kJ/mol.




Similar content being viewed by others
REFERENCES
G. E. Adams and F. C. Bishop, “An experimental investigation of thermodynamic mixing properties and unit–cell parameters of forsterite–monticellite solid solutions,” Am. Mineral. 70, 714–722 (1985).
C. Brousse, R. C. Newton, and O. J. Kleppa, “Enthalpy of formation of forsterite, enstatite, akermanite, monticellite and merwinite at 1073 K determined by alkali borate solution calorimetry,” Geochim. Cosmochim. Acta 48, 1081–1088 (1984).
A. Chopelas, “Single crystal Raman spectra of forsterite, fayalite, and monticellite,” Am. Mineral. 76, 1101–1109 (1991).
N. V. Chukanov, Infrared Spectra of Mineral Species: Exte-nded Library (Springer–Verlag GmbH, Dordrecht–Heidelberg–New York–London, 2014).
D. A. Duke and J. D. Stephens, “Infrared investigations of the olivine group minerals,” Am. Mineral. 49, 1388–1406 (1964).
W. P. Griffith, “Raman studies on rock–forming minerals. Part I. Orthosilicates and cyclosilicates,” J. Chem. Soc. (A), 1372–1377 (1969).
M. Handke, K. Kosinsky, and P. Tarte, “Vibrational spectra and force constants calculations of the isotopic species of MgCaSiO4,” J. Mol. Struct. 115, 401–404 (1984).
T. J. B. Holland, “Dependence of entropy on volume for silicate and oxide minerals review and a predictive model,” Am. Mineral. 74, 5–13 (1989).
T. J. B. Holland, and R. Powell, “An enlarged an updated internally consistent thermodynamic dataset with uncertainties and correlations: the system K2O–Na2O–CaO–MgO–MnO–FeO–Fe2O3–Al2O3–TiO2–SiO2–C–H2–O2,” J. Metamorph. Geol. 8, 89–124 (1990).
T. J. B. Holland and R. Powell, “An inrernally consistent thermodynamic data set for phases of petrological interest,” J. Metamorph. Geol. 16, 309–343 (1998).
T. J. B. Holland and R. Powell, “An improved and extended inrernally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids,” J. Metamorph. Geol. 29, 333–383 (2011).
I. A. Kiseleva, “Thermodynamic properties and stability of pyrope,” Geokhimiya, No. 6, 845–854 (1976).
I. A. Kiseleva, L. P. Ogorodova, N. D. Topor, and O. G. Chigareva, “Thermochemical study of the CaO–MgO–SiO2 system,” Geokhimiya, No. 12, 1811–1825 (1979).
L. Liu, “The high–pressure phase transformations of monticellite and implications for upper mantle mineralogy,” Phys. Earth Planet. Int. 20, 25–29 (1979).
K. Mohanan, S. K. Sharma, and F. C. Bishop, “A Raman spectral study of forsterite–monticellite solid solutions,” Am. Mineral. 78, 115–121 (1993).
T. Mouri, and M. Enami “Raman spectroscopic study of olivine–group minerals,” J. Mineral. Petrol. Sci. 103, 100–104 (2008).
G. B. Naumov, B. N. Ryzhenko, and I. L. Khodakovsky, Reference Book on Thermodynamic Valyes (For Geologists) (Atomizdat, Moscow, 1971) [in Russian].
A. Navrotsky, and W. J. Coons, “Thermochemistry of some pyroxenes and related compounds,” Geochim. Cosmochim. Acta 40, 1281–1295 (1976).
S. N. Nenasheva and A. A. Agakhanov, “New Data on Minerals from the Shishim Mine, Shishim Mounts, South Urals, Russia,” New Data on Minerals 51, 45–52 (2016).
K. J. Neuvonen, “Heat of formation of merwinite and monticellite,” Am. J. Sci. (Bowen Vol.), 373–380 (1952).
H. Onken,“Verfeinerung der kristallstruktur von monticellit,“ Tscher. Miner. Petrog.10 (1–4), 34–44 (1965).
T. Pilati, F. Demartin, and C. M. Gramaccioli, “Thermal parameters for minerals of the olivine group: their implication on vibrational spectra, thermodynamic functions and transferable force fields,” Acta Crystallogr. B51, 721–733 (1995).
B. Piriou and P. McMilan, “The high–frequency vibrational spectra of vitreous and crystalline orthosilicates,” Am. Mineral. 68, 426–443 (1983).
R. A. Robie and B. S. Hemingway, “Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures,” U.S. Geol. Surv. Bull. 2131, (1995).
R. A. Robie, B. S. Hemingway, and J. R Fisher, “Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures,” U.S. Geol. Surv. Bull. 1452, (1978).
RRUFF Database of Raman Spectroscopy, X–ray Diffraction and Chemistry of Minerals. http://www.rruff. info/
Z. D. Sharp, E. J. Essene, L. M. Anovitz, G. W. Metz, E. F. Westrum, Jr., B. S. Hemingway, and J. W. Valley, “The heat capacity of a natural monticellite and phase equilibria in the system CaO–MgO–SiO2–CO2,” Geochim. Cosmochim. Acta 50, 1475–1484 (1986).
Z. D. Sharp R. M. Hazen, and L. W. Finger, “High–pressure crystal chemistry of monticellite, CaMgSiO4,” Am. Mineral. 72, 748–755 (1987).
Yu.V. Shvarov, “HCh: New potentialities for the thermodynamic simulation of geochemical systems offered by Windows”, Geochemistry International 46, 834–839 (2008).
V. I. Sinyakov and N. M. Sinyakova, Monticellite skarns of Gornaya Shoria, Zap. Vsesoyuz. Mineral. O-va, No. 6, 720–727 (1961).
K. A. Subbotin, L. D. Iskhakova, E. V. Zharikov, and S. V. Lavrishchev, “Investigation of the crystallization features, atomic structure, and microstructure of chromium–doped monticellite,” Crystallogr. Rep. 53 (7), 1107–1111 (2008).
R. D. Warner, and W. C. Luth, “Two–phase data for the join montichellite (CaMgSiO4)–forsterite (Mg2SiO4): experimental results and numerical analysis,” Am. Mineral. 58, 998–1008 (1973).
V. A. Zharikov, K. I. Shmulovich, and V. K. Bulatov, “Experimental studies in the system CaO–MgO–Al2O3–SiO2–CO2–H2O and conditions of high-temperature metamorphism,” Tectonophysics 43, 145–162 (1977).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Kurdyukov
Rights and permissions
About this article
Cite this article
Ogorodova, L.P., Gritsenko, Y.D., Vigasina, M.F. et al. Thermodynamic Properties of Montecellite. Geochem. Int. 57, 1343–1348 (2019). https://doi.org/10.1134/S0016702919120085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702919120085