Abstract
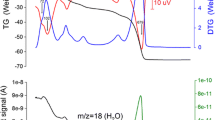
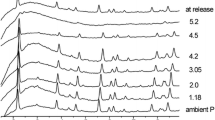
Structural changes during dehydration and subsequent decomposition in thaumasite Ca3Si(SO4)(CO3)(OH)6·12 H2O were studied by in situ synchrotron powder diffraction between 303 and 1,098 K. Evolution of the crystal structure was observed through 28 structure refinements, by full profile Rietveld analysis performed in the P63 space group, between 300 and 417 K, whereupon the thaumasite structure was observed to breakdown. Within this temperature range, the cell parameters of thaumasite increased as a function of temperature in a nearly linear fashion up to about 393 K, at which temperature, a slight slope change was observed. Above 400 K, the thermogravimetric analysis revealed that the dehydration process proceeded very rapidly while the refined occupancy of water molecules dropped below a critical level, leading to instability in the thaumasite structure. At a same time, a remarkable change in the unit cell parameters occurring at about 417 K indicated that the crystal structure of thaumasite collapsed on losing the crystallization water and it turned amorphous. This result indicated that the dehydration/decomposition of thaumasite was induced by the departure of the crystallization water. At about 950 K, anhydrite and cristobalite crystallized from the thaumasite glass.








Similar content being viewed by others
References
Angel RJ, Finger LW, Hazen RM, Kanzaki M, Weidner DJ, Liebermann RC, Veblen DR (1989) Structure and twinning of single-crystal MgSiO3 garnet synthesized at 17 Gpa and 1800°C. Am Mineral 74:509–512
Barnett SJ, Adam CD, Jackson ARW (2000) Solid solutions between ettringite, Ca6Al2(SO4)3 (OH)12·26 H2O, and thaumasite Ca3SiSO4CO3(OH)6·12 H2O. J Mater Sci 35:4109–4114
Bensted J (1999) Thaumasite—background and nature in deterioration of cements, mortars and concretes. Cem Concr Compos 21:117–121
Brough AR, Atkinson A (2001) Micro-Raman spectroscopy of Thaumasite. Cem Concr Compos 31:421–424
Carpenter AB (1963) Oriented overgrowths of Thaumasite on Ettringite. Am Miner 48:1394–1396
Crammond NJ (1985) Thaumasite in failed cement mortars and renders from exposed brickwork. Cem Concr Res 15:1039–1050
Drábik M, Gáliková L (2003) Method of thermal analysis in the detection of thaumasite and its presence in the sulphate-attacked concrete. Solid State Phenomena 90–91:33–38
Edge RA, Taylor FW (1971) Crystal structure of Thaumasite, [Ca3Si(OH)6·12 H2O](SO4)(CO3). Acta Cryst B27:594–601
Effenberger H, Kirfel A, Will G, Zobetz E (1983) A further refinement of the crystal structure of thaumasite, Ca3Si(OH)6CO3 SO4·12 H2O. N Jb Miner Mh 2:60–68
Federico M (1970) Un inconsueto deposito di thaumasite fra i tufi del cratere di Prata Porci (Colli Albani). Miner Mag 32:567–572
Fei Y (1995) AGU reference shelf 2: mineral physics and crystallography—a handbook of physical constants. In: Ahrens TJ (ed) AGU, Washington, pp 29–44
Font-Altaba M (1960) A thermal study of thaumasite. Mineral Mag 32:567–572
Frost DJ, Fei Y (1998) Stability of phase D at high pressure and high temperature. J Geophys Res 103:7463–7474
Giampaolo C (1986) Dehydration kinetics of thaumasite at ambient pressure. N Jb Miner Mh Jg H 3:126–134
Granger MM, Protas J (1969) Détermination et étude de la structure crystalline de la jouravskite Ca3MnIV(SO4)(CO3)(OH)6·12 H2O. Acta Crystallogr B 25:1943–1951
Grubessi O, Mottana A, Paris E (1986) Thaumasite from the Tschwinning [N’Chwaning] mine, South Africa. Tschermaks Mineral Petrog Mitt 35:149–156
Horiuchi H, Hirano M, Ito E, Matsui Y (1982) MgSiO3 (ilmenitetype): single-crystal X-ray diffraction study. Am Mineral 67:788–793
Horiuchi H, Ito E, Weidner DJ (1987) Perovskite-type MgSiO3: single-crystal X-ray diffraction study. Am Mineral 72:357–360
Hurlbut CS, Baum JL (1960) Ettringite from Franklin, New Jersey. Am Mineral 45:1137–1143
Jacobsen SD, Smyth JR, Swope RJ (2003) Thermal expansion of hydrated six-coordinate silicon in thaumasite, Ca3Si(OH)6 (CO3) (SO4) 12 H2O. Phys Chem Miner 30:321–329
Kirov GN, Poulieff CN (1968) On the infra-red spectrum and thermal decomposition products of thaumasite, Ca3H2(CO3/SO4)SiO4·13 H2O. Mineral Mag 36:1003–1011
Knill DC (1960) Thaumasite from County Down, Northern Ireland. Mineral Mag 32:416–418
Lachaud R (1979) Thaumasite and ettringite in construction materials. Annales de l’Institut du Batiment et des Travaux Publics 32:370–373
Larson AC, Von Dreel RB (2000) GSAS general structure analysis system. Report LAUR. Los Alamos National Laboratory, Los Alamos, pp 86–748
McDonald AM, Peterson OV, Gault RA, Johnsen O, Niedermayr G, Branstätter F (2001) Micheelsenite, (Ca, Y)3Al(PO3OH, CO3)(CO3)(OH)6·12 H2O, a new mineral from Mont Saint-Hilaire, Quebec, Canada and the Nanna pegmatite, Narsaarsuup Qaava, South Greenland. Neues Jahrbuch Mineralogie Monatshefte 8:337–351
Merlino S, Orlandi P (2001) Carraraite and zaccagnaite, two new minerals from the Carrara marble quarries: their chemical compositions, physical properties, and structural features. Am Mineral 86:1293–1301
Noack Y (1983) Occurrence of thaumasite in a seawater–basalt interaction, Mururoa atoll (French Polynesia, South Pacific). Mineral Mag 47:47–50
Norby P (1997) Synchrotron powder diffraction using imaging plates: crystal structure determination and Rietveld refinement. J Appl Cryst 30:21–30
Peters JJ (1984) Triassic Traprock minerals of New Jersey. Rocks Miner 59:157–183
Reeder RJ (1983) Crystal chemistry of the rhombohedral carbonates. In: Reeder RJ (ed) Carbonates: mineralogy and chemistry. Reviews in mineralogy, vol 11. Mineralogical Society of America, pp 1–47
Ross NL, Shu JF, Hazen RM (1990) High-pressure crystal chemistry of stishovite. Am Mineral 75:739–747
Vogt T (1938) Thaumasite from Sulitelma, Norway. Norsk Geol Tidskr 18:291–303
Yang H, Prewitt CT, Frost DJ (1997) Crystal structure of the dense hydrous magnesium silicate, phase D. Am Mineral 82:651–654
Zemann J, Zobetz E (1981) Do the carbonate groups in thaumasite have anomalously large deviations from coplanarity? Kristallografija 26:1215–1217
Acknowledgments
We are indebted to Carlo Meneghini (University of Rome) and Marco Merlini (University of Milan) for their assistance during the experiments at the BM08 (GILDA) beamline (ESRF, Grenoble, France) and the processing of the Translating Imaging Plate data. We thank the referees for the careful reviews and for many useful comments and suggestions. The Italian CNR and INFM are also acknowledged for providing financial support to GILDA and its associated facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martucci, A., Cruciani, G. In situ time resolved synchrotron powder diffraction study of thaumasite. Phys Chem Minerals 33, 723–731 (2006). https://doi.org/10.1007/s00269-006-0124-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0124-8