Abstract
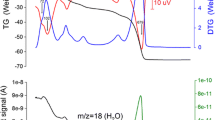
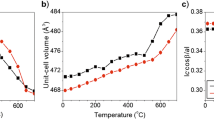
The thermal response of the natural ferroan phlogopite-1M, K2(Mg4.46Fe0.83Al0. 34Ti0.22)(Si5.51Al2. 49)O20[OH3.59F0.41] from Quebec, Canada, was studied with an in situ neutron powder diffraction. The in situ temperature conditions were set up at −263, 25, 100°C and thereafter at a 100°C intervals up to 900°C. The crystal structure was refined by the Rietveld method (R p=2.35–2.78%, R wp=3.01–3.52%). The orientation of the O–H vector of the sample was determined by the refinement of the diffraction pattern. With increasing temperature, the angle of the OH bond to the (001) plane decreased from 87.3 to 72.5°. At room temperature, a = 5.13 Å, b = 9.20 Å, c = 10.21 Å, β = 100.06° and V(volume) = 491.69 Å3. The expansion rate of the unit cell dimensions varied discontinuously with a break at 500°C. The shape of the M-octahedron underwent some significant changes such as flattening at 500°C. At temperatures above 500°C, the octahedral thickness and mean <M–O> distance was decreased, while the octahedral flattening angle increased. Those results were attributed to the Fe oxidation and dehydroxylation processes. The dehydroxylation mechanism of the ferroan phlogopite was studied by the Fourier transform infrared spectroscopy (FTIR) after heated at temperatures ranging from 25 to 800°C with an electric furnace in a vacuum. In the OH stretching region, the intensity of the OH band associated with Fe2+(N B-band) begun to decrease outstandingly at 500°C. The changes of the IR spectra confirmed that dehydroxylation was closely related to the oxidation in the vacuum of the ferrous iron in the M-octahedron. The decrease in the angle of the OH bond to the (001) plane, with increasing temperature, might be related to the imbalance of charge in the M-octahedra due to Fe oxidation.






Similar content being viewed by others
References
Cagliotti G, Paoletti A, Ricci F (1958) Choice of collimators for a crystal spectrometer for neutron diffraction. Nucl Instrum 3:223–228
Catti M, Ferraris G, Hull S, Pavese, A (1994) Powder neutron diffraction study of 2M 1 muscovite at room pressure and at 2 GPa. Eur J Miner 6:171–178
Chon C-M, Kim SA, Moon H-S (2003) Crystal structure of biotite at high temperatures and heat-treated biotite using neutron powder diffraction. Clays Clay Miner 51:519–528
Dollase WA (1986) Correction of intensities for preferred orientation in powder diffractometry: application of the march model. J Appl Cryst 19:267–272
Giese RF (1984) Electrostatic energy models of micas. Mineralogical Society of America. Rev Miner 13:105–144
Guggenheim S, Chang Y-H, Koster Van Groos AF (1987) Muscovite dehydroxylation: high-temperature studies. Am Miner 72:537–550
Hawthorne FC, Welch MD, Ventura GD, Liu S, Robert J-L, Jenkins DM (2000) Short-range order in synthetic aluminuous tremolites: an infrared and triple-quantum MAS NMR study. Am Miner 85:1716–1724
Hazen RM, Burnham CW (1973) The crystal structure of one-layer phlogopite and annite. Am Miner 58:889–900
Hogg CS, Meads RE (1975) A Mössbauer study of thermal decomposition of biotites. Min Mag 40:79–88
Joswig W (1972) Neutronenbeugungsmessungen an einem 1 M-Phlogopit. Neues Jahrb Min Monatsh 1–11
Mookerjee M, Redfern SAT, Zhang M (2001) Thermal response of structure and hydroxyl ion of phengite-2M 1: an in situ neutron diffraction and FTIR study. Eur J Miner 13:545–555
Pavese A, Ferraris G, Prencipe M, Ibberson R (1997) Cation site ordering in phengite 3 T from the dora-maira massif (western alps): a variable-temperature neutron powder diffraction study. Eur J Miner 9:1183–1190
Pavese A, Ferraris G, Pischedda V, Ibberson R (1999) Tetrahedral order in thermodynamic consequences. Eur J Miner 11:309–320
Pavese A, Ferraris G, Pischedda V, Radaelli P (2000) Further study of the cation ordering in phengite 3T by neutron powder diffraction. Min Mag 64:11–18
Pavese A, Ferraris G, Pischedda V, Fauth F (2001) M1-site occupancy in 3T and 2M(1) phengites by low temperature neutron powder diffraction: reality or artifact? Eur J Miner 13:1071–1078
Rancourt DG, Christie IAD, Lamarche G, Swainson I, Flandrois S (1994) Magnetism of synthetic and natural annite mica: ground state and nature of excitations in an exchange-wise two-dimensional easy-plane ferromagnet with disorder. J Magn Magn Mater 138:31–44
Rayner JH (1974) The crystal structure of phlogopite by neutron diffraction. Min Mag 39:850–856
Redhammer GJ, Beran A, Schneider J, Amthauer G, Lottermoser W (2000) Spectroscopic and structural properties of synthetic micas on the annite-siderophyllite binary: synthesis, crystal structure refinement, Mössbauer, and infrared spectroscopy. Am Miner 85:449–465
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Cryst 2:65–71
Rodriguez-Carvajal J (1998) FullProf: Rietveld profile matching and integrated intensity refinement of X-ray and neutron data (PC-version). Version 3.5d
Russell RL, Guggenheim S (1999) Crystal structures of near-end-member phlogopite at high temperatures and heat-treated Fe-rich phlogopite: the influence of the O, OH, F site. Can Miner 37:711–729
Sanz J, González-Carreňo, Gancedo R (1983) On dehydroxylation mechanisms of a biotite in vacuo and in oxygen. Phys Chem Miner 9:14–18
Takeda H, Donnay JDH (1966) Trioctahedral one-layer micas. III. Crystal structure of a synthetic lithium fluormica. Acta Cryst 20:638–646
Takeda H, Morosin B (1975) Comparison of observed and predicted structural parameters of mica at high temperature. Acta Cryst B31:2444–2452
Tripathi RP, Chandra U, Chandra R, Lokanathan S (1978) A Mössbauer study of the effects of heating biotite, phlogopite and vermiculite. J Inorg Nucl Chem 40:1293–1298
Tutti F, Dubrovinsky LS, Nygren M (2000) High-temperature study and thermal expansion of phlogopite. Phys Chem Miner 27:599–603
Vedder W (1964) Correlations between infrared spectrum and chemical compostion of mica. Am Miner 49:736–768
Vedder W, Wilkins RWT (1969) Dehydroxylation and rehydroxylation, oxidation and reduction of micas. Am Miner 54:482–509
Wilkins RWT (1967) The hydroxyl-stretching region of the biotite mica spectrum. Min Mag 36:325–333
Young RA (1993) The rietveld method. International Union of Crystallography, Oxford University Press, Oxford, p 29
Acknowledgements
This research was supported by the Basic Research Project of the Korea Atomic Energy Research Institute (KAERI) and the Korea Institute of Geoscience and Mineral Resources (KIGAM) funded by the Ministry of Science and Technology of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chon, CM., Lee, CK., Song, Y. et al. Structural changes and oxidation of ferroan phlogopite with increasing temperature: in situ neutron powder diffraction and Fourier transform infrared spectroscopy. Phys Chem Minerals 33, 289–299 (2006). https://doi.org/10.1007/s00269-005-0045-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-005-0045-y