Abstract
Aim
To compare the efficacy and safety of synthetic and biological meshes in ventral hernia repair (VHR) and abdominal wall reconstruction (AWR).
Methods
We screened all clinical trials that reported the application of synthetic and biological meshes in VHR and AWR using Medline, Web of Science, and Embase (Ovid). Only comparative studies with similar baselines such as age, sex, body mass index, degree of wound contamination, and hernia defects between the intervention and control groups were included. Effect sizes with 95% confidence were pooled using a random- or fixed-effects model based on the size of heterogeneity. A sensitivity analysis was performed to test the stability of the results.
Results
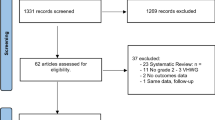
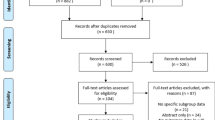
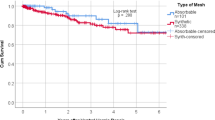
Ten studies with 1305 participants were included. Biological meshes were associated with significantly higher recurrence rate (OR, 2.09; 95% CI 1.42–3.08; I2 = 50%), surgical site infection (OR, 1.47; 95% CI 1.10–1.97; I2 = 30%), higher re-admission rate (OR, 1.51; 95% CI 1.05–2.17; I2 = 50%), and longer length of hospital stay (SMD, 0.37; 95% CI 0.10–0.65; I2 = 72%). Similar surgical site occurrence, re-operation rate, and mesh explantation rate were observed among biological and synthetic meshes. Biological meshes have no difference in recurrence rate as compared to synthetic meshes, between the clean-contaminated, and contamination-infected fields (OR, 1.41; 95% CI 0.41–4.87 vs 3.00; 95% CI 1.07–8.46; P = 0.36).
Conclusion
Synthetic meshes are a safe alternative to biological meshes for VHR and AWR. Considering the high cost of biological meshes, synthetic meshes are more appropriate for the VHR and AWR.





Similar content being viewed by others
Data availability
Not applicable. No datasets were generated during the production of this work.
References
Schlosser KA, Renshaw SM, Tamer RM, Strassels SA, Poulose BK (2023) Ventral hernia repair: an increasing burden affecting abdominal core health. Hernia 27(2):415–421. https://doi.org/10.1007/s10029-022-02707-6
Nguyen MT, Berger RL, Hicks SC, Davila JA, Li LT, Kao LS, Liang MK (2014) Comparison of outcomes of synthetic mesh vs suture repair of elective primary ventral herniorrhaphy: a systematic review and meta-analysis. JAMA Surg 149(5):415–421
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg (Chicago, Ill. : 1960) 133(4):378–382.
Halm JA, de Wall LL, Steyerberg EW, Jeekel J, Lange JF (2007) Intraperitoneal polypropylene mesh hernia repair complicates subsequent abdominal surgery. World J Surg 31(2).
Rosen MJ, Krpata DM, Ermlich B, Blatnik JA (2013) A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 257(6):991–996
Slater NJ, van der Kolk M, Hendriks T, van Goor H, Bleichrodt RP (2013) Biologic grafts for ventral hernia repair: a systematic review. Am J Surg 205(2):220–230
Hiles M, Record Ritchie RD, Altizer AM (2009) Are biologic grafts effective for hernia repair?: a systematic review of the literature. Surg Innovat 16(1):26–37.
Harris HW, Primus F, Young C, Carter JT, Lin M, Mukhtar RA, Yeh B, Allen IE, Freise C, Kim E, Sbitany H, Young DM, Hansen S (2021) Preventing recurrence in clean and contaminated hernias using biologic versus synthetic mesh in ventral hernia repair: the PRICE randomized clinical trial. Ann Surg 273(4):648–655
Rosen MJ, Krpata DM, Petro CC, Carbonell A, Warren J, Poulose BK, Costanzo A, Tu C, Blatnik J, Prabhu AS (2022) Biologic vs synthetic mesh for single-stage repair of contaminated ventral hernias: a randomized clinical trial. JAMA Surg 157(4):293–301
Miserez M, Lefering R, Famiglietti F, Mathes T, Seidel D, Sauerland S, Korolija D, Heiss M, Weber G, Agresta F, Steup W-H, Śmietański M, Ribeiro R, Cuccurullo D, Catena F, Rudroff C, Rosanelli G, Schön F, Smet B, Wenger F, Saad S, Naver L, Neugebauer E (2021) Synthetic versus biological mesh in laparoscopic and open Ventral Hernia Repair (LAPSIS): results of a multinational, randomized, controlled, and double-blind trial. Ann Surg 273(1):57–65
Morales-Conde S, Hernández-Granados P, Tallón-Aguilar L, Verdaguer-Tremolosa M, López-Cano M (2022) Ventral hernia repair in high-risk patients and contaminated fields using a single mesh: proportional meta-analysis. Hernia J Hernias Abdominal Wall Surg 26(6):1459–1471
Morris MP, Mellia JA, Christopher AN, Basta MN, Patel V, Qiu K, Broach RB, Fischer JP (2021) Ventral hernia repair with synthetic mesh in a contaminated field: a systematic review and meta-analysis. Hernia J Hernias Abdominal Wall Surg 25(4):1035–1050.
Chamieh J, Tan WH, Ramirez R, Nohra E, Apakama C, Symons W (2017) Synthetic versus biologic mesh in single-stage repair of complex abdominal wall defects in a contaminated field. Surg Infect 18(2):112–118
Herrero A, Gonot Gaschard M, Bouyabrine H, Perrey J, Picot MC, Guillon F, Fabre JM, Souche R, Navarro F (2022) Comparative study of biological versus synthetic prostheses in the treatment of ventral hernias classified as grade II/III by the Ventral Hernia Working Group. J Visceral Surg 159(2):98–107.
Koscielny A, Widenmayer S, May T, Kalff J, Lingohr P (2018) Comparison of biological and alloplastic meshes in ventral incisional hernia repair. Langenbecks Arch Surg 403(2):255–263
Liang MK, Berger RL, Nguyen MT, Hicks SC, Li LT, Leong M (2014) Outcomes with porcine acellular dermal matrix versus synthetic mesh and suture in complicated open ventral hernia repair. Surg Infect 15(5):506–512
Majumder A, Winder JS, Wen Y, Pauli EM, Belyansky I, Novitsky YW (2016) Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery 160(4):828–838
Olavarria OA, Bernardi K, Dhanani NH, Lyons NB, Harvin JA, Millas SG, Ko TC, Kao LS, Liang MK (2021) Synthetic versus biologic mesh for complex open ventral hernia repair: a pilot randomized controlled trial. Surg Infect 22(5):496–503
Shao JM, Ayuso SA, Deerenberg EB, Elhage SA, Prasad T, Colavita PD, Augenstein VA, Heniford BT (2022) Biologic mesh is non-inferior to synthetic mesh in CDC class 1 & 2 open abdominal wall reconstruction. Am J Surg 223(2):375–379
Sanders D, Lambie J, Bond P, Moate R, Steer JA (2013) An in vitro study assessing the effect of mesh morphology and suture fixation on bacterial adherence. Hernia 17(6):779–789. https://doi.org/10.1007/s10029-013-1124-5
Pérez-Köhler B, Fernández-Gutiérrez M, Pascual G, García-Moreno F, San Román J, Bellón JM (2016) In vitro assessment of an antibacterial quaternary ammonium-based polymer loaded with chlorhexidine for the coating of polypropylene prosthetic meshes. Hernia J Hernias Abdominal Wall Surg 20(6):869–878.
Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YW, Rosen MJ (2012) In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg Offic J Soc Surg Aliment Tract 16(11):2139–2144
Katzen M, Ayuso SA, Sacco J, Ku D, Scarola GT, Kercher KW, Colavita PD, Augenstein VA, Heniford BT (2023) Outcomes of biologic versus synthetic mesh in CDC class 3 and 4 open abdominal wall reconstruction. Surg Endosc 37(4):3073–3083
Baumann DP, Butler CE (2012) Bioprosthetic mesh in abdominal wall reconstruction. Semin Plast Surg 26(1):18–24
De Silva GS, Krpata DM, Gao Y, Criss CN, Anderson JM, Soltanian HT, Rosen MJ, Novitsky YW (2014) Lack of identifiable biologic behavior in a series of porcine mesh explants. Surgery 156(1):183–189
Bellows CF, Wheatley BM, Moroz K, Rosales SC, Morici LA (2011) The effect of bacterial infection on the biomechanical properties of biological mesh in a rat model. PLoS ONE 6(6):e21228
Cole WC, Balent EM, Masella PC, Kajiura LN, Matsumoto KW, Pierce LM (2015) An experimental comparison of the effects of bacterial colonization on biologic and synthetic meshes. Hernia J Hernias Abdominal Wall Surg 19(2):197–205.
Atema JJ, de Vries FEEE, Boermeester MA (2016) Systematic review and meta-analysis of the repair of potentially contaminated and contaminated abdominal wall defects. Am J Surg 212(5).
Acknowledgements
There are no acknowledgments.
Funding
This work is supported by the National Natural Science Foundation of China (Number: 81970455, 82170526).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, H., Wang, R., Dong, W. et al. Synthetic Versus Biological Mesh in Ventral Hernia Repair and Abdominal Wall Reconstruction: A Systematic Review and Recommendations from Evidence-Based Medicine. World J Surg 47, 2416–2424 (2023). https://doi.org/10.1007/s00268-023-07067-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-023-07067-5