Abstract
Background
Ischemia and reperfusion injury is an important factor that determines graft function after liver transplantation, and oxygen plays a crucial role in this process. However, the relationship between the intraoperative high fraction of inspiratory oxygen (FiO2) and living-donor-liver-transplantation (LDLT) outcome remains unclear.
Patients and Methods
A total of 199 primary adult-to-adult LDLT cases in Kyoto University Hospital between January 2010 and December 2017 were enrolled in this study. The intraoperative FiO2 was averaged using the total amount of intraoperative oxygen and air and defined as the calculated FiO2 (cFiO2). The cutoff value of cFiO2 was set at 0.5.
Results
Between the cFiO2 <0.5 (n = 156) and ≥0.5 group (n = 43), preoperative recipients’ background, donor factors, and intraoperative parameters were almost comparable. Postoperatively, the cFiO2 ≥0.5 group showed a higher early allograft dysfunction (EAD) rate (P = 0.049) and worse overall graft survival (P = 0.036) than the cFiO2 <0.5 group. Although the cFiO2 ≥0.5 was not an independent risk factor for EAD in multivariable analysis (OR 2.038, 95%CI 0.992–4.186, P = 0.053), it was an independent risk factor for overall graft survival after LDLT (HR 1.897, 95%CI 1.007–3.432, P = 0.048).
Conclusion
The results of this study suggest that intraoperative high FiO2 may be associated with worse graft survival after LDLT. Avoiding higher intraoperative FiO2 may be beneficial for LDLT recipients.
Similar content being viewed by others
Introduction
In liver transplantation, ischemia and reperfusion injury (IRI) is one of the important factors that determine postoperative graft function [1]. The abrupt cessation of blood flow and subsequent ischemia decreases several antioxidants, such as glutathione, and also tissue adenosine triphosphate [2,3,4]. This ischemic phase itself damages the liver tissue [4]; moreover, after reperfusion, numerous cellular and molecular factors in various pathways are rapidly activated, which further aggravate the liver injury [1]. The ultimate consequence of this process is apoptotic or necrotic cell death, graft dysfunction, and graft loss [1]. From the findings of previous studies, it is now becoming clear that oxygen and its free radicals, called reactive oxygen species, play an important role in this process [5, 6].
Oxygen therapy was once regarded as a harmless treatment option [7]; however, several studies conducted in the field of critical care medicine have demonstrated that too much oxygenation could be harmful to the human body and that the restriction of oxygen supply would improve the outcomes of critically ill patients [8, 9]. Among patients admitted to the intensive care unit (ICU) following resuscitation of cardiac arrest, Kilgannon et al. showed that the first partial pressure of arterial oxygen (PaO2) after ICU admission equal to or more than 300 mmHg was independently associated with increased in-hospital mortality [10]. Therefore, avoiding excessive oxygen supply is now becoming a new standard for patients’ care [8].
Based on these findings, we hypothesized that excessive oxygen supply before and after graft reperfusion may influence the severity of IRI and the outcome after liver transplantation; however, this topic still remains unexplored. From the above, the aim of this study was to investigate the impact of higher intraoperative fraction of inspiratory oxygen (FiO2) on the outcome after living-donor-liver-transplantation (LDLT).
Patients and Methods
Study Design
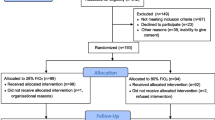
A total of 219 adult (≥18 years old) patients underwent primary LDLT in Kyoto University Hospital from January 2010 through December 2017. Among them, patients with posterior segment graft (n = 8), who died within seven days of LDLT (n = 2) and with preoperative pulmonary complications (hepato-pulmonary syndrome and moderate to severe pulmonary hypertension or intrapulmonary arteriovenous shunt, n = 7) were excluded. In addition, patients with incomplete operation records (n = 3) were also excluded. Ultimately, 199 patients were enrolled in this study.
The donor–recipient selection criteria, detailed surgical procedure, and regimens of postoperative immunosuppression, including blood type incompatible liver transplantation, have been described previously [11,12,13]. In our institute, the anesthesiology team is responsible for the intraoperative circulatory and respiratory care of patients. Although the target of intraoperative FiO2 was set at approximately 0.4, the anesthesiologists were free to control the FiO2 at their discretion.
All study protocols were approved by the Ethics Committee of Kyoto University (Approval number: R1473-4), and all procedures were conducted in accordance with the Declaration of Helsinki of 1996.
Data Collection
Data were retrospectively collected from patient charts. The preoperative recipient demographic data collected were age, sex, status prior to surgery (hospitalized or ICU stay), etiology of liver disease, Model for End-stage Liver Disease (MELD) score, hepatorenal syndrome, percent vital capacity (%VC), forced expiratory volume in one second/forced vital capacity (FEV1/FVC ratio), and results of blood sample tests. The donor demographic data collected were age, graft-to-recipient weight ratio (GRWR), blood type–incompatible donor, and graft type. Intraoperative parameters included operation time, blood loss, cold and warm ischemia time, portal vein pressure (PVP), intraoperative splenectomy and total volume of infusion. Postoperative parameters included in-hospital mortality, result of blood culture, acute cellular and humoral rejection within one year after LDLT, early allograft dysfunction (EAD) rate, and causes of graft loss. Postoperative platelet count until postoperative day (POD) 42 and bilirubin level until POD 7 were also collected.
The intraoperative FiO2 usually fluctuated during the operation; therefore, to precisely assess the difference of intraoperative FiO2 between patients, the value was averaged using the total amount of intraoperative oxygen (O2) and air administered, i.e., the calculated FiO2 (cFiO2) was defined as {O2(L) + Air(L) × 0.21}/{O2(L) + Air(L)}.
Recipients with %VC ≥80% and FEV1/FVC ratio ≥70% were classified as having normal pulmonary function. There were 23 cases of recipients (15 and 8 cases for cFiO2 <0.5 and ≥0.5 group, respectively) whose preoperative pulmonary functions were not measured due to the patient’s condition.
The results of arterial blood gas analyses including the PaO2, partial pressure of arterial carbon dioxide (PaCO2), HCO3−, and PaO2/FiO2 (P/F) ratio were obtained at three time points during operation: at the start, within 30 min before or after portal reperfusion, and at the end of the operation.
Recipients’ preoperative skeletal muscle mass index (SMI), visceral to subcutaneous adipose tissue area ratio (VSR), and intra-muscular adipose tissue content (IMAC) were also collected, because our team previously reported that preoperative low SMI, high IMAC, and high VSR, i.e., “positive 3 body composition markers,” are independent risk factors for mortality after LDLT [14].
EAD was defined by the presence of one or more of the following: (i) total bilirubin ≥10 mg/dL on POD 7, (ii) prothrombin time-international normalized ratio ≥1.6 on POD 7, and (iii) aspartate aminotransferase or alanine aminotransferase ≥2000 IU/mL within the first seven postoperative days [15].
Parameters Analyzed
First, the distribution of cFiO2 was evaluated and the factors associated with the difference of cFiO2 between patients were assessed. Second, based on the previous study [16], patients were divided according to the cutoff value of the cFiO2 “0.5.” Subsequently, the background and postoperative outcomes of patients were evaluated. Third, the prognostic factors associated with EAD were evaluated using univariable and multivariable analyses. The data included in the univariable analysis were determined according to the previous reports [17, 18]. Although the previous study has shown that a high preoperative bilirubin level is a risk factor for EAD [17], in this analysis, the clearance of total bilirubin level during the first seven days after LDLT, defined as “(preoperative bilirubin–POD7 bilirubin)/preoperative bilirubin,” was used instead of a simple preoperative bilirubin level. Finally, the postoperative survival curves of recipients with the cFiO2 <0.5 and ≥0.5 were compared. Subsequently, the prognostic factors associated with overall graft survival were analyzed using univariable and multivariable analyses. The data included in the univariable analysis were also determined according to the previous reports [14, 19,20,21,22,23].
Statistical Analysis
Continuous data were expressed as the median and interquartile range (IQR) and were compared using the Wilcoxon rank-sum test. Categorical data were expressed as counts, and percentages and were compared using the Fisher’s exact test. The overall graft survival rate was calculated using the Kaplan–Meier method, and differences between curves were evaluated using the log-rank test. Variables with P <0.10 in the univariable analysis were considered candidates for multivariable logistic regression analysis or Cox regression analysis. The results of the multivariable analysis are shown as odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals (CIs). According to the previous recommendation of the American Statistical Association [24], we avoided describing P <0.05 as “statistically significant”; instead, we described the P values as continuous quantities in the text, figures, and tables. All statistical analyses were performed using JMP Pro, version 14.0.0 (SAS Institute, Inc., Cary, NC).
Results
Distribution of cFiO2 and the Factors Associated with the Variance
The cFiO2 showed a bimodal distribution, and the peaks were found around 0.4 and 0.5 (Fig. 1); therefore, we evaluated the factors associated with the difference of the cFiO2 between patients (Fig. 2). First, we divided the patients according to the P/F ratio at the start of the operation (Fig. 2A and B); however, both the P/F ratio ≥300 and ≥400 groups had a peak around cFiO2 0.5 and the P/F ratio <400 group also showed a peak around cFiO2 0.4. Patients were also divided according to their pulmonary function (Fig. 2C), preoperative status (Fig. 2D), MELD score (Fig. 2E), and PaO2 at the start of the operation (Fig. 2F); however, none seemed to influence the variance of cFiO2. Finally, we also assessed the trend of the cFiO2 during the observational period. As shown in Fig. 3, no apparent chronological changes in cFiO2 were found during this observational period.
Evaluation of the factors associated with the variance of the cFiO2. The distribution of cFiO2 was divided according to the P/F ratio (≥or <300 and 400, A and B, respectively), recipients’ pulmonary function (C), recipients’ preoperative status (D), MELD score (E), and PaO2 at the start of the operation (F). The normal pulmonary function was defined as recipients with %VC ≥80% and FEV1/FVC ratio ≥70%. Calculated FiO2 (cFiO2) was defined as {O2(L) + Air(L) × 0.21}/{O2(L) + Air(L)}. FEV1/FVC, forced expiratory volume in one second/forced vital capacity; FiO2, fraction of inspiratory oxygen; MELD, model for end-stage liver disease; PaO2, partial pressure of arterial oxygen; P/F, PaO2/FiO2 ratio; %VC, percent vital capacity
Summary of Patients’ Demographic Data (Table 1 )
Recipients were divided into those with the cFiO2 <0.5 (n = 156) and ≥0.5 (n = 43). Both groups showed almost comparable preoperative recipients’ background, donor factors, and intraoperative parameters. Although the P values of recipients’ preoperative alanine aminotransferase and intraoperative warm ischemia time were less than 0.05, the difference in actual values was almost clinically negligible. Postoperatively, the cFiO2 ≥0.5 group showed a trend toward higher EAD rate than the cFiO2 <0.5 group.
Figure 4 shows the results of arterial blood gas analyses during the operation. PaO2 was first comparable between the two groups; however, the cFiO2 ≥0.5 group showed higher PaO2 during portal reperfusion and at the end of the operation compared to the cFiO2 <0.5 group (Fig. 4A). The levels of PaCO2 and HCO3− were almost similar between the two groups throughout the operation (Fig. 4B and C). The P/F ratio was first lower in the cFiO2 ≥0.5 group; however, the values became almost comparable thereafter (Fig. 4D).
The results of arterial blood gas analysis during operation. The cFiO2 ≥0.5 group showed higher PaO2 at the portal reperfusion and at the end of the operation compared to the cFiO2 <0.5 group (A). The results of PaCO2 (B) and HCO3− (C) were almost similar between the two groups throughout the operation. The P/F ratio was initially lower in the cFiO2 ≥0.5 group; however, the value became almost comparable between the two groups during portal reperfusion (D). Calculated FiO2 (cFiO2) was defined as {O2(L) + Air(L) × 0.21}/{O2(L) + Air(L)}. FiO2, fraction of inspiratory oxygen; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; P/F, PaO2/FiO2 ratio
Comparisons of the postoperative platelet count and total bilirubin values are shown in Fig. 5. The cFiO2 ≥0.5 group showed a trend toward lower platelet count after POD5 (Fig. 5A) and higher bilirubin levels after POD3 (Fig. 5B).
Comparisons of postoperative platelet count and total bilirubin value. The cFiO2 ≥0.5 group showed a trend toward lower platelet count after POD5 (A) and higher bilirubin levels after POD 3 (B). Calculated FiO2 (cFiO2) was defined as {O2(L) + Air(L) × 0.21}/{O2(L) + Air(L)}. FiO2, fraction of inspiratory oxygen
Risk Factor Analysis for EAD
Univariable analysis revealed that recipient age, gender, donor age, left lobe graft, and the cFiO2 ≥0.5 were potential risk factors for EAD after LDLT (Table 2). Multivariable analysis showed that the male recipients (P = 0.048) and donor age (P = 0.023) were independent risk factors for EAD. Although the P value did not reach the statistical threshold, the cFiO2 ≥0.5 was considered as a possible risk factor for EAD (P = 0.053).
Graft Survival After LDLT and Risk Factor Analysis
Figure 6 shows the overall graft survival after LDLT. The cFiO2 ≥0.5 group showed worse graft survival than the cFiO2 <0.5 group. Table 3 shows the results of the risk factor analysis for overall graft survival after LDLT. Univariable analysis revealed that the positive 3 body composition markers, donor age ≥40 years, GRWR <0.6% blood type incompatible donor, and the cFiO2 ≥0.5 were potential risk factors for graft loss after LDLT. Multivariable analysis showed that the positive 3 body composition markers (P <0.001), blood type incompatible donor (P = 0.046), and the cFiO2 ≥0.5 (P = 0.048) were independent risk factors for overall graft survival after LDLT.
Discussion
The results of this study demonstrated that high intraoperative FiO2 may be independently associated with worth graft outcome after LDLT. This is also confirmed by the fact that the cFiO2 ≥0.5 group showed higher EAD rate, although the cFiO2 ≥0.5 was not shown to be an independent factor in multivariable analysis.
In a previous experimental study, it was shown that perioperative hyperoxic conditions worsen liver IRI. Using a mouse partial liver ischemia–reperfusion model, Zangl et al. demonstrated that the mice under postoperative hyperoxic (FiO2 = 0.6) conditions showed higher glutamate-pyruvate-transaminase level, reactive oxygen species, and histological injury score than the mice under normoxic (FiO2 = 0.21) conditions [25]. Subsequently, they showed that the harmful effects of high FiO2 were ameliorated by depletion of granulocytes or Kupffer cells or by knocking out of the p47phox unit of the NADPH-oxidase [25]. Considering that granulocytes and Kupffer cells are the main sources of reactive oxygen species, their results suggested that oxygen is a very important factor that affects IRI and that restriction of oxygen supply may decrease the severity of liver injury after ischemia–reperfusion.
In our study, the cFiO2 ≥0.5 groups showed a worse overall graft survival after LDLT. Regarding short-term outcomes, the cFiO2 ≥0.5 group was also associated with a higher rate of EAD. Univariable analysis showed that higher cFiO2 was a potential risk factor for EAD; however, it did not reach the statistical threshold for independent risk factor in multivariable analysis. This may be because the difference in cFiO2 between the two groups (median 0.44 for the cFiO2 <0.5 group and 0.53 for the cFiO2 ≥0.5 group, respectively) was not strong enough to detect the difference in the EAD rate. If we could have compared the FiO2 0.3 and 0.8, which were used in several prospective studies [26,27,28], the difference in short-term outcomes might have appeared more clearly. Liver transplantation is a life-saving procedure, and recipients are usually critically ill; therefore, it would be ethically difficult to conduct a prospective study allocating patients into extremely different intraoperative FiO2. We think the results of this study suggested the possibility that high intraoperative FiO2 might increase the EAD rate and could lead to worse graft outcomes after LDLT.
A lower P/F ratio at the start of the operation would be the main reason for the higher oxygen supply in the cFiO2 ≥0.5 group (Fig. 4D). However, in the cFiO2 ≥0.5 group, the P/F ratio improved thereafter and the PaO2 increased during portal reperfusion than in the cFiO2 <0.5 group (Fig. 4A). Resolving of atelectasis by positive pressure ventilation or removal of a large amount of ascites or pleural effusion may be associated with improved oxygenation in the cFiO2 ≥0.5 group. As shown in the Supplementary Figure, spot FiO2 was gradually decreased during operation even in the cFiO2 ≥0.5 group; however, the difference was smaller than that observed in the cFiO2 <0.5 group; therefore, the FiO2 became relatively higher than expected, and the liver grafts were exposed to a higher oxygen environment in the cFiO2 ≥0.5 group. These results suggest that intraoperative FiO2 can be reduced safely in some patients, which may lead to the suppression of IRI, lower EAD rate, and improved outcome after LDLT.
Our study has several limitations. First, the study was retrospective and conducted in a single institution. Our results should be confirmed in multicenter prospective studies. Second, it would have been more informative if we could have assessed all possible parameters in our institute during risk factor analysis for EAD and graft survival. However, due to the limited number of patients, we selected the factors included in the analysis, according to the results of previous studies. Further accumulation of cases is needed for a more accurate investigation. Last, we have to be careful about applying the results of this study to the patients undergoing LDLT. We think that these results do not mean the intraoperative FiO2 should be kept “lower as possible.” Intraoperative FiO2 should be adjusted according to the condition of each patient and critically ill patients sometimes need higher than usual oxygen to maintain a normal oxygenation level. However, as is shown in this study, even the most of the patients in cFiO2 <0.5 group showed PaO2 over 120 mmHg and those in cFiO2 >0.5 group showed further higher PaO2 during LDLT; therefore, we think that FiO2 could be reduced safely for these patients. From the above, we think that our results should be interpreted as “avoiding unnecessarily high intraoperative FiO2 in LDLT.”
In conclusion, this retrospective study suggested that intraoperative high FiO2 might increase EAD and have some impact on graft survival after LDLT. Avoiding unnecessarily high intraoperative oxygenation may have some beneficial effects in patients undergoing LDLT.
References
Abu-Amara M, Yang SY, Tapuria N et al (2010) Liver ischemia/reperfusion injury: processes in inflammatory networks–a review. Liver Transpl 16:1016–1032
Churchill TA, Cheetham KM, Fuller BJ et al (1994) Glycolysis and energy metabolism in rat liver during warm and cold ischemia: evidence of an activation of the regulatory enzyme phosphofructokinase. Cryobiology 31:441–452
Kurokawa T, Kobayashi H, Nonami T et al (1996) Mitochondrial glutathione redox and energy producing function during liver ischemia and reperfusion. J Surg Res 66:1–5
Fukai M, Hayashi T, Yokota R et al (2005) Lipid peroxidation during ischemia depends on ischemia time in warm ischemia and reperfusion of rat liver. Free Radic Biol Med 38:1372–1381
Wu MY, Yiang GT, Liao WT et al (2018) Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem 46:1650–1667
Jaeschke H, Smith CV, Mitchell JR (1988) Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest 81:1240–1246
Habre W, Petak F (2014) Perioperative use of oxygen: variabilities across age. Br J Anaesth 113(Suppl 2):ii26–ii36
O’Driscoll BR, Howard LS, Davison AG (2008) BTS guideline for emergency oxygen use in adult patients. Thorax 63(Suppl 6):vi1–vi68
Six S, Jaffal K, Ledoux G et al (2016) Hyperoxemia as a risk factor for ventilator-associated pneumonia. Crit Care 20:195
Kilgannon JH, Jones AE, Shapiro NI et al (2010) Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 303:2165–2171
Tanaka K, Ogura Y, Kiuchi T et al (2004) Living donor liver transplantation: eastern experiences. HPB 6:88–94
Morioka D, Egawa H, Kasahara M et al (2007) Outcomes of adult-to-adult living donor liver transplantation: a single institution’s experience with 335 consecutive cases. Ann Surg 245:315–325
Kaido T, Ogawa K, Fujimoto Y et al (2013) Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 13:1549–1556
Hamaguchi Y, Kaido T, Okumura S et al (2018) Proposal for new selection criteria considering pre-transplant muscularity and visceral adiposity in living donor liver transplantation. J Cachexia Sarcopenia Muscle 9:246–254
Wadei HM, Lee DD, Croome KP et al (2016) Early allograft dysfunction after liver transplantation is associated with short- and long-term kidney function impairment. Am J Transplant 16:850–859
Hovaguimian F, Lysakowski C, Elia N et al (2013) Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 119:303–316
Pomposelli JJ, Goodrich NP, Emond JC et al (2016) Patterns of early allograft dysfunction in adult live donor liver transplantation: the A2ALL experience. Transplantation 100:1490–1499
Song JL, Yang J, Yan LN et al (2017) A new index predicts early allograft dysfunction following living donor liver transplantation: a propensity score analysis. Dig Liver Dis 49:1225–1232
Uemura T, Wada S, Kaido T et al (2016) How far can we lower graft-to-recipient weight ratio for living donor liver transplantation under modulation of portal venous pressure? Surgery 159:1623–1630
Kubota T, Hata K, Sozu T et al (2018) Impact of donor age on recipient survival in adult-to-adult living-donor liver transplantation. Ann Surg 267:1126–1133
Yao S, Kaido T, Uozumi R et al (2018) Is portal venous pressure modulation still indicated for all recipients in living donor liver transplantation? Liver Transpl 24:1578–1588
Yao S, Kaido T, Yagi S et al (2019) Impact of imbalanced graft-to-spleen volume ratio on outcomes following living donor liver transplantation in an era when simultaneous splenectomy is not typically indicated. Am J Transplant 19:2783–2794
Kamo N, Kaido T, Hammad A et al (2015) Impact of elderly donors for liver transplantation: a single-center experience. Liver Transpl 21:591–598
Wasserstein RL, Lazar NA (2016) The ASA statement on p-values: context, process, and purpose. Am Stat 70:129–133
Zangl Q, Martignoni A, Jackson SH et al (2014) Postoperative hyperoxia (60%) worsens hepatic injury in mice. Anesthesiology 121:1217–1225
Belda FJ, Aguilera L, García de la Asunción J et al (2005) Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA 294:2035–2042
Bickel A, Gurevits M, Vamos R et al (2011) Perioperative hyperoxygenation and wound site infection following surgery for acute appendicitis: a randomized, prospective, controlled trial. Arch Surg 146:464–470
García-Botello SA, García-Granero E, Lillo R et al (2006) Randomized clinical trial to evaluate the effects of perioperative supplemental oxygen administration on the colorectal anastomosis. Br J Surg 93:698–706
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
YM participated in research design, paper writing, and data analysis. TK revised the draft critically for important intellectual content. MH made substantial contributions to the design of the work and analyzed data. MS made substantial contributions to the design of the work and analyzed data. MM made substantial contributions to the design of the work and analyzed data. SY made substantial contributions to the design of the work and analyzed data. NK made substantial contributions to the design of the work and analyzed data. SK made substantial contributions to the design of the work. SY revised the draft critically for important intellectual content. SU made substantial contributions to the design of the work and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
All authors declared no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethical Approval
All study protocols were approved by the Ethics Committee of Kyoto University (Approval number: R1473-4), and all procedures were conducted in accordance with the Declaration of Helsinki of 1996.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
268_2022_6544_MOESM1_ESM.eps
Changes in spot FiO2 during operation. Although the difference was smaller than that observed in the cFiO2 <0.5 group, the spot FiO2 gradually decreased during operation even in the cFiO2 ≥0.5 group. Calculated FiO2 (cFiO2) was defined as {O2(L) + Air(L) × 0.21}/{O2(L) + Air(L)}. FiO2, fraction of inspiratory oxygen (EPS 133 KB)
Rights and permissions
About this article
Cite this article
Miyachi, Y., Kaido, T., Hirata, M. et al. Intraoperative High Fraction of Inspiratory Oxygen is Independently Associated with Worse Outcome After Living-Donor Liver Transplantation: A Retrospective Study. World J Surg 46, 1776–1787 (2022). https://doi.org/10.1007/s00268-022-06544-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06544-7