Abstract
This study examined the relative influence of nutrients (nitrogen and phosphorus) and habitat on algal biomass in five agricultural regions of the United States. Sites were selected to capture a range of nutrient conditions, with 136 sites distributed over five study areas. Samples were collected in either 2003 or 2004, and analyzed for nutrients (nitrogen and phosphorous) and algal biomass (chlorophyll a). Chlorophyll a was measured in three types of samples, fine-grained benthic material (CHLFG), coarse-grained stable substrate as in rock or wood (CHLCG), and water column (CHLS). Stream and riparian habitat were characterized at each site. TP ranged from 0.004–2.69 mg/l and TN from 0.15–21.5 mg/l, with TN concentrations highest in Nebraska and Indiana streams and TP highest in Nebraska. Benthic algal biomass ranged from 0.47–615 mg/m2, with higher values generally associated with coarse-grained substrate. Seston chlorophyll ranged from 0.2–73.1 μg/l, with highest concentrations in Nebraska. Regression models were developed to predict algal biomass as a function of TP and/or TN. Seven models were statistically significant, six for TP and one for TN; r 2 values ranged from 0.03 to 0.44. No significant regression models could be developed for the two study areas in the Midwest. Model performance increased when stream habitat variables were incorporated, with 12 significant models and an increase in the r 2 values (0.16–0.54). Water temperature and percent riparian canopy cover were the most important physical variables in the models. While models that predict algal chlorophyll a as a function of nutrients can be useful, model strength is commonly low due to the overriding influence of stream habitat. Results from our study are presented in context of a nutrient-algal biomass conceptual model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutrients, particularly nitrogen and phosphorus, have long been identified as a major water-quality issue because of their role in the eutrophication of streams, lakes, and coastal waters (Carpenter and others 1998; U.S. Environmental Protection Agency [EPA] 1998; NRC 2000). The U.S. Environmental Protection Agency (U.S. EPA 2000) cited nutrients as the third leading cause of water-quality impairment in surface waters. Of the 23% of the total river and stream miles assessed by States and Tribes, 30% were impaired because of nutrient enrichment. More recently, nitrogen and phosphorus were identified as two of the four most common stressors in streams in the United States (U.S. EPA 2006), with riparian disturbance and streambed sediments the other two dominant stressors. Although sources of nutrients are highly varied, agriculture is commonly associated with excessive nutrient loading to streams. The EPA identified agriculture as the leading source of pollution in the assessed streams of the Nation, contributing to 48% of the reported water-quality problems in impaired streams (U.S. EPA 2002). The U.S. Geological Survey’s (USGS) National Water-Quality Assessment (NAWQA) Program generally found higher nitrogen and phosphorus concentrations in agricultural streams than in other settings (Fuhrer and others 1999). In response to the myriad of studies that identify nutrients as having major ecological and economic consequences, the U.S. EPA proposed using regional nutrient criteria for various surface-water types (U.S. EPA 1998).
Although the ecological effects of excess nutrients are often discussed in regard to lakes and coastal waters (Turner and Rabalais 1994; Rabalais and others 1996; Brezonik and others 1999), nutrient enrichment also negatively influences streams and rivers. Nutrients commonly cause an increase in algal biomass, which can result in increased diel swings in oxygen concentrations thereby stressing some aquatic species (Correll 1998). If filamentous green-algae or macrophyte biomass is substantial, fine-grained sediments can be trapped and thereby alter natural habitat (Sand-Jensen 1998; Wharton and others 2006). The above factors often lead to a decrease in diversity and native species composition (Welch 1992; Carpenter and others 1998; Smith and others 1999), with some exotic species able to exploit the altered condition.
Empirical regression models that predict algal biomass as a function of nutrients are often used for establishing nutrient concentrations that are protective of stream conditions. Although many of these models are statistically significant, nutrients commonly explain only a small to moderate amount of the variation in algal biomass. For example, Dodds and others (2002) reported that nitrogen and/or phosphorus concentrations only accounted for 10–40% of the variation in benthic algal biomass. A primary reason for the weak relationship between algal biomass and nutrients in stream environments is the complex interactions of physical and biological factors (Biggs 1996a, b; Clausen and Biggs 1998). Habitat variables found to limit algal production include light limitation due to canopy shading (Mosisch and others 2001) and turbidity (Munn and others 1989), water temperature (Kilkus and others 1975; Munn and others 1989) and hydrologic disturbance (Powers 1992; Biggs 1995; Riseng and others 2004). Pesticides also have been reported to alter algal biomass in agricultural streams (Kosinski 1984), as have biological factors, such as grazing by invertebrates or fish (Lamberti and Resh 1983; Powers 1992).
To date, there have been no studies comparing nutrient and algal biomass interactions across major agricultural regions and the effect habitat has in modifying these relationships. In this study we: (1) compare and contrast the concentrations of nutrients and algal biomass among five agricultural areas, (2) determine the relationship between the concentrations of nutrients and algal biomass, and (3) determine the relative influence of physical habitat on nutrient-biomass relationships. Results from this study will be assessed in light of the Nutrient-Algal Biomass Concept.
Methods
Study Areas
This study was conducted in five study areas characterized by extensive agricultural land use (Fig. 1, Table 1). The five areas included the Columbia Plateau (CCYK) and Central Nebraska (CNBR), which were sampled in 2003, and the Georgia Coastal Plain (GCP), Delmarva Peninsula (DLMV), and the White Miami (WHMI), which were sampled in 2004. The GCP and DLMV were sampled in May and June, with the CCYK, CNBR, and WHMI sampled in July and August. All five study areas contain extensive agricultural lands, ranging from an average of 26% in the CCYK to 90% in the WHMI (Table 1); however, individual study areas vary substantially in percent agriculture and the intensity of agricultural practices. Eastern study areas (GCP, DLMV, and WHMI) are humid, with agriculture relying primarily on natural rainfall; average drainage basin size ranged from 14.5 to 145.9 km2, and average canopy cover was 61–88% (Table 1). In contrast, western study areas (CCYK and CNBR) are in more arid environments that rely on irrigation practices; streams had larger average drainage basins (443–652 km2) and less average canopy cover (22–28%).
Site Selection
Sites were selected within each study area to maximize the potential range of nutrient concentrations while minimizing other natural or anthropogenic factors. Sites were not selected in order to extrapolate to the population of streams in the region. Basin-level coverages within a study area were derived from 30-m digital elevation model (DEM) data obtained from the USGS Elevation Derivatives for National Applications (EDNA) project. In order to minimize variability because of stream size, we initially selected all independent drainage basins between 100 and 400 km2 as candidate basins, although the final selection included a greater range in basin size. The initial selection of sites relied partially on modeled estimates of nitrogen and phosphorus loading to each of the independent basins. National-scale analysis of the NAWQA data has demonstrated that nitrogen loading to the land surface was significantly related to nitrogen yields to streams (Fuhrer and others 1999) and possibly could be used as a surrogate for nutrient concentration in streams with sparse water-quality data. The nutrient input estimates used during this analysis were derived from county-level fertilizer sales, atmospheric deposition, and livestock data (Ruddy and others 2006). Final selection of sites was based upon modeled nutrient loading to a basin and existing nutrient data. This resulted in the selection of 28–30 wadeable sites within each study area that spans the greatest range in nutrient concentrations as possible within a study area.
Habitat
Physical habitat was assessed at the stream reach scale (ca. 150 m), which was defined as a repetition of a geomorphic sequence (e.g., 2 riffles and 2 pools), or 20 channel widths if repetitive units were not present within the reach (Fitzpatrick and others 1998). A total of 11 equidistant transects oriented perpendicular to the longitudinal axis of the channel were established throughout the reach, with wetted channel width (m) measured at each transect. Water depth (cm), water velocity (cm/s), and percent substrate type (bedrock, boulder, cobble, gravel, sand, and silt) were measured at five points across each transect. A densiometer was used at each transect to measure percent canopy cover at the center channel. Reach gradient was determined from water-surface elevations measured with a surveyor’s level. Additional field measurements included stream discharge (m3/s) and water temperature (°C).
Along with measuring instantaneous discharge at all sites, we used two other flow measurements. In order to assess streamflow conditions prior to our study we calculated a flow metric from 13 long-term continuous gages (10% of total sites) distributed across the five study areas. The metric used was the maximum daily streamflow for 30 days prior to sampling divided by the median streamflow over 5–15 previous years. This metric reflects the extent to which flows during the prior 30 days exceeded the long-term median flows, with a value of greater than 3 reflecting a physical disturbance that can alter benthic habitat (Clausen and Biggs 1998).
A Base Flow Index (BFI) was also calculated for all sites as an indicator of annual flow patterns. Base-flow index (BFI) values for ungaged streams were estimated by computing the basin-average value of a BFI geospatial raster dataset (Wolock 2003a) for the drainage basin of the ungaged site. The BFI raster dataset was derived through interpolation of BFI point values estimated for USGS stream gages (Wolock 2003b). These point values for stream gages were calculated using an automated hydrograph separation technique that partitions each value in a time series of measured daily streamflow into slowly varying (base flow) and rapidly varying (quick flow) components. Base flow is commonly assumed to originate from ground-water discharge into the stream. The computer program used to estimate base flow was developed by the U.S. Bureau of Reclamation (Wahl and Wahl 1995) and is commonly referred to as the BFI Program. This program estimates the annual base-flow volume and computes an annual base-flow index, which is the ratio of base flow to total flow for a given period. The BFI index values used in this study are the average values for the period of record at the sites. While the method is not expected to precisely quantify the amount of base flow in a stream, the BFI has been found to be a reasonable indicator of differences in base flow among different streams (Wahl and Wahl 1995). A BFI value of 0 indicates all flow comes from surface water and a value of 1 all flow comes from groundwater.
Water Chemistry
Nutrient samples were collected twice at each site; the first sample was collected ca. 30 days prior to the algal sampling and the second was collected at the same time as the algal samples. Nutrient samples were collected using a depth- and width-integrated sampling method (Shelton 1994). Samples collected for analyses of dissolved constituents were filtered in the field with a 0.45-μm pore-size capsule filter; samples for total constituents were unfiltered. All nutrient samples were placed on wet ice, shipped to the USGS National Water Quality Laboratory in Arvada, Colorado, where they were analyzed within 24-h. Suspended sediment samples were collected during the second nutrient sample.
Samples were analyzed for nitrate (NO3), nitrite (NO2), ammonium (NH3), dissolved and total organic N (DON and TON), orthophosphate (OP), and total phosphorus (TP). Nutrients were analyzed using colorimetric methods; NH3 plus DON and TON, and TP by microkjeldahl digestion (Patton and Truitt 2000); NH3 by salicylate hypochlorite (Fishman 1993); NO2 by diazotization; NO3 plus NO2 by Cd reduction; and OP by phosphomolybdate (Fishman 1993). Dissolved inorganic nitrogen (DIN) was calculated by summing the concentrations of NO3, NO2, and NH3. Total nitrogen was determined using either the alkaline persulfate digestion (Patton and Kryskalla 2003) or by summing nitrogen species. For purposes of statistical analysis, all non-detect values were given one half of the detection limit, and then TN, DIN, TP, and OP values were averaged for the two sampling periods. Suspended sediment samples were analyzed for total suspended sediment (mg/l) by the USGS sediment laboratory.
Algal Biomass
Chlorophyll a was measured in the seston (CHLS) and on both coarse-grained rock or wood substrate (CHLCG) and fine-grained benthic sediments (CHLFG). For the CHLCG samples, a single reach-scale (100–200 m) composite algal sample was collected with the collection technique varying by substrate type (Moulton and others 2002). For rock substrates, algae were scraped from five rocks collected throughout the length of the reach, with all individual rock samples within a reach composited. Snags (submerged logs or smaller woody debris) were sampled by scraping a known area from five pieces of wood collected throughout the reach with samples also composited. The CCYK and WHMI collected CHLCG from rock substrate, and the GCP, CNBR, and DLMV collected CHLCG samples from wood. Fine-grained algal biomass (CHLFG) samples were collected and processed using a modified method by Stevenson and Stoermer (1981). Five fine-grained samples were collected from throughout the reach using an inverted Petri plate with samples from a site composited. Elutriation was used to separate the algae from the fine grained material by adding 100 mL of tap water, capping, and inverting 15 times. The sample was permitted to sit for 5 s and then the algal-water mixture was decanted. This process was repeated two more times. Ten mL of the homogenized mixture was then withdrawn and filtered (Moulton and others 2002). This step was repeated until a thin pigmented film was present on the filter. Samples were filtered onto 47-mm glass fiber filters and shipped on dry ice. Water-column samples (CHLS) were collected by depth- and width-integrated methods and filtered through a Whatman GF/F 47-mm glass fiber filter, which was then wrapped in foil and placed on dry ice (Moulton and others 2002). Chlorophyll was analyzed using the acidified fluorometic method (Arar and Collins 1997).
Basin/Riparian Land Use
Basin-scale geographic measures were compiled using the Environmental Systems Research Institute’s (ESRI) Arc Info Workstation geographic information system (GIS). All raster processing took place at 30-m resolution. The source for land-cover information was an enhanced version (Nakagaki and Wolock 2005) of the USGS National Land Cover Data 1992 (Vogelmann and others 2001). The 1:100,000-scale National Hydrography Dataset (U.S. Geological Survey and U.S. Environmental Protection Agency 2003) was the source for the streams data. Riparian variables were determined at the reach and segment scale using methods outlined in Johnson and Zelt (2005). An additional riparian variable describing the sum of all habitat types in the riparian zone within 25 m of the stream also was determined.
Statistical Analyses
ANOVA combined with Tukey’s multicomparison tests were used to determine if there were significant differences in nutrient or chlorophyll among study areas. Nutrient and chlorophyll data were log10 transformed and all statistics run using Systat© (Systat Software, version 11, 2004). Paired t-tests were used to determine if there was a significant difference between chlorophyll a based upon analyses of CHLCG or CHLFG samples.
Linear regression was used to predict the three chlorophyll a measures based upon TN and DIN or TP and OP (Table 2). The influence of habitat on the chlorophyll-nutrient models was assessed using multiple regression models (Table 2); with some habitat variables normalized using the log10 or square root transformations. Canopy (CAN) and percent fine-grained sediment (FG) could not be normalized, and therefore were treated as categorical variables in the model.
Results
Environmental Characteristics
While streams in the five study areas shared many similar features, there were differences related to basin size, canopy cover, and suspended sediment (Table 1). Basin size was greatest in western study areas with the CCYK having the largest average basin (652 km2), whereas the smallest basins were found in the DMLV. Streams in the CCYK and CNBR generally had less riparian canopy cover averaging 25%; whereas, streams in the eastern study areas commonly averaged 61–88% cover by study area. Suspended sediment concentrations were highest in the CNBR with an average of 158 mg/l; lower suspended sediment concentrations were found in the eastern study areas. Results from the long-term continuous flow gages indicated that 2 of the 13 sites (15%) had flows that exceeded 3 times the median flow for the 30 days prior to sampling, indicating that algal biomass could have been influenced at some sites by high flows prior to sampling. One gage was in each of the DMLV and GCP, however all other gages in the DMLV and GCP did not show the high flow event, indicating that the influence of high flows was minimal and localized. The CCYK, CNBR and WHMI had no sites where the flow metric exceeded the high flow event level.
Nutrient and Algal Biomass
Concentrations of TN ranged from 0.15 to 21.2 mg/l across all five areas; TN concentrations were significantly lower in CCYK and GCP—1.6 and 1.1 mg/l, respectively—than in the other three areas (Table 3). DIN followed a similar pattern. TP ranged from 0.004 to 2.7 mg/l, with the CNBR having significantly higher concentrations (0.72 mg/l) and GCP significantly lower concentrations (0.04 mg/l). TP concentrations in the CCYK, WHMI, and DLMV were similar. Concentration patterns of OP were similar to those of TP. Benthic algal biomass varied depending on the study area and habitat sampled (Table 3). Coarse-grain algal biomass (CHLCG), which includes both rock and wood, ranged from 0.47 to 615 mg/m2. Lowest average values were associated with wood in the GCP (2.65 mg/m2) and highest average values reported for wood in the DLMV (98.9 mg/m2). Algal biomass on rock was not significantly different between CCYK and WHMI. There was a wide range in chlorophyll values on fine-grained substrate (CHLFG); significantly lower average values were observed in the DLMV (18.8 mg/m2) and GCP (9.8 mg/m2). The highest average CHLFG value was found in the CNBR (77 mg/m2); however, there was no significant difference in fine-grain algal biomass among the CCYK, CNBR, and WHMI areas. CHLS concentrations ranged from 0.2 to 73.1 μg/l, with an average of 7.9 μg/l. The CNBR had significantly higher CHLS concentrations, but there were no significant differences in CHLS concentrations among the other four study areas.
Nutrient-Algal Biomass Model
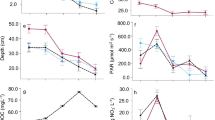
The use of regression analysis to predict algal biomass as a function of TN and TP concentrations resulted in 7 significant models (P < 0.05, Table 4). Only TN and TP were used in our models because OP and DIN added little to the performance of the models and TN and TP are the focus of nutrient criteria. In general, TP was a better predictor of algal biomass than TN, with CHLS and CHLFG producing more models than CHLCG. When all five study areas were combined (ALL), there was one significant model for each of the three chlorophyll sample types; however, the CHLCG and CHLFG models had low r 2 value and therefore are of limited use. The CHLS-TP model for all study areas combined had the highest r 2 (0.44). Only four study-area based nutrient-biomass models were found to be significant, with TP the independent variable (Table 4). These four models included CHLS-TP in the GCP, CHLFG-TP in the CCYK (negative relationship), and CHLFG-TP and CHLS-TP in the DLMV. No significant regression models could be developed for the CNBR and WHMI. The relationship between TP and CHLFG illustrates how associations can vary among regions (Fig. 2). There was a weak relationship between CHLFG and TP when ALL data were combined (r 2 = 0.12; Fig. 2a). Models of TP and CHLFG for individual study areas (Fig. 2b-f) ranged from positive (DLMV, r 2 = 0.32) to negative (CCYK, r 2 = 0.20), but no significant relationship was found in the other three study areas (GCP, CNBR, WHMI).
Nutrient-Algal Biomass-Habitat Models
The inclusion of habitat into the chlorophyll-nutrient models resulted in a greater number of significant models and generally higher r 2 values (P < 0.05; r 2 = 0.16–0.54; Table 5). As with the nutrient-only models, TN was the significant nutrient for predicting CHLCG, and TP was the significant nutrient for predicting both CHLFG and CHLS. The physical habitat variables that were important predictors in the ALL regression models included canopy (CAN, 3 models); temperature (TEMP, 2 models) and percent fine-grain substrate (FG, 2 models); slope (SLOPE), velocity (VEL), and base-flow index (BFI) all in one model each. The individual study area-based models also improved with the addition of habitat variables, with some models explaining a higher percentage of the variance in chlorophyll. The habitat variables that were significant predictors in one or more models included TEMP, CAN, BFI, SLOPE, and VEL. Of the nine study area models that were found to be significant, six included only habitat variables.
Because canopy was found to be an important variable, piecewise regression was used to determine if there was a significant breakpoint in percent canopy cover for chlorophyll. Results indicated that for CHLCG the breakpoint was at 63% canopy cover, and for CHLFG the breakpoint was at 40% canopy cover; there was no significant breakpoint for CHLS. Therefore, we divided the sites into two groups on the basis of percentage of canopy cover. As expected, open canopy streams (CAN <50%) had significantly greater benthic algal biomass and seston chlorophyll concentrations than closed canopy streams (CAN >50%) (Table 6). Furthermore, for CHLCG and CHLFG, there was a negative correlation between TP and TN concentrations and algal biomass in open streams (CAN <50%), whereas in more canopied systems (CAN >50%) TP and TN were positively correlated with biomass (Table 7). CHLS concentrations were positively correlated with both TP and TN under both scenarios with no difference between open and closed streams (Table 7).
Discussion
Nutrients and Chlorophyll
Agricultural streams pose a unique set of challenges for assessing nutrient-biota interactions because of elevated nutrient concentrations combined with the alteration of stream and riparian habitat. Agricultural streams are known to contain some of the highest nutrient concentrations in any land-use setting (U.S. EPA 2002; Fuhrer and others 1999; Mueller and Spahr 2006), which complicates the design of a nutrient gradient study because of the paucity of sites with low nutrient concentrations. In regards to TP, our study captured a broad TP gradient (0.004–2.69 mg/l) with 15% of sites having average concentrations below levels (ca 0.03 mg/l) reported to elicit increases in algal biomass (Stevenson and others 2006; Dodds and others 2002). However, the CNBR and WHMI contained no sites with TP concentrations below the 0.03 mg/l level. The ability to analyze a gradient for TN was more problematic in that only 6% of sites had concentrations below levels (ca. 0.5 mg/l) that are reported to elicit a growth response (Stevenson and others 2006; Dodds and others 2002), with the CNBR and WHMI again having no sites with concentrations below 0.5 mg/l.
While there is no standard biomass value that is considered excessive, Welch and others (1988) reported that benthic algal chlorophyll a values above 100 mg/m2 are considered potentially problematic. Benthic chlorophyll a in our study ranged from 0.47 to 615 mg/m2, with 13% of the sites having values greater than 100 mg/m2. We also found that chlorophyll a could be greater on either hard substrate or fine-grained sediment depending on the study area. Biggs and Shand (1987) reported that benthic algal biomass was approximately 15 times greater on hard substrate (e.g., rock) than on fine-grained material due to the influence of substrate stability. The high chlorophyll a on fine-grain bed material in our study is because sampling occurred during a more stable flow period.
Nutrient-Algal Biomass Models
Benthic and seston chlorophyll are commonly used as key response variables to increases in concentrations of TN and TP, with the assumption that any increase in TN and/or TP results in an increase in algal biomass. This study found that with all sites combined, our benthic chlorophyll models were significant but had low r 2 values (0.03 for CHLCG and 0.12 for CHLFG). The CHLS model had an r 2 value of 0.44, however this is due to suspended algae containing both chlorophyll and nutrients (van Nieuwenhuyse and Jones 1996). Results from the literature are mixed, ranging from models showing no relationship between nutrients and benthic algal biomass (Munn and others 1989; Kjeldsen 1994) to others with r 2 values ranging from 0.05 to 0.6 (Biggs and Close 1989; Lohman and others 1992; Dodds and others 2002).
The finding that only two of the five study areas were found to have a significant model for benthic algal biomass demonstrates the challenge in addressing nutrient criteria in agricultural settings. While the DLMV did show the predicted positive association between CHLFG and TP, the CCYK relationship was negative. The negative relationship in the CCYK is due in part to the positive correlation of TP and suspended sediment (r = 0.67) with elevated suspended sediment potentially interfering with benthic algae by decreasing light penetration and/or by sediment deposition. The inability to develop a significant benthic model for the remaining three study areas is because of the limited range of nutrient concentrations at the high or low end. Dodds and others (2002) and Stevenson and others (2006) reported that benthic algae had a TP threshold of ca. 0.03 mg/l and a TN threshold of ca. 0.5 mg/l. The average concentration of TP in the GCP was 0.036 mg/l with a range of 0.01–0.06 mg/l, therefore TP may not have been high enough to elicit a strong response in algal growth. In contrast, the CNBR and WHMI study areas had no significant TP models because both study areas contained elevated TP concentrations, with even the lowest nutrient concentrations well above threshold values. The lack of any significant TN study-area-based models is also because the high concentrations of TN. While large-scale studies like that of Dodds and others (2002) often show statistical relationships between nutrient concentrations and benthic chlorophyll, these relationships do not always hold up at basin to regional scales.
Nutrient-Biomass-Habitat Models
The accrual (colonization plus growth) of benthic algal biomass is a function of nutrients, light, and temperature, whereas hydrologic stability and grazing control the process of biomass loss (Biggs 1996a). Therefore, physical and biological disturbance commonly reduce biomass in the stream to levels comparable to an earlier period. When reach-level habitat variables were added to the models there was a consistent improvement in model performance and an increase in the number of significant models. The most frequently incorporated habitat variables were water temperature (TEMP) and percent canopy cover (CAN), and to a lesser extent water velocity (VEL) and the base flow index (BFI).
Temperature is a key variable for the development of algal biomass because it regulates the rate of cellular metabolism and growth, and has been demonstrated to be an important limiting factor (Munn and others 1989; Bowes and others 2007). In this study, water temperature had a positive influence in four of the 12 models, but played the most critical role in the GCP and CNBR study areas.
Various measures of stream-flow are known to be important to the accrual and loss of algal biomass. Jowett and Biggs (1997) reported that benthic algal biomass increased up to moderate water velocities, but then decreased when water velocities increased further. Our study found that water velocity was an important variable in three of the models (ALL CHLFG, GCP CHLCG, and DLMV CHLS), but consistently had a negative influence on algal biomass. Given that this study was conducted during more stable flow periods, velocity would be more limiting during high flow events, particularly in regards to benthic algae on fine-grained substrates.
Hydraulic stability is also known to be a major determinant in the growth of algal biomass, with hydraulic stability over periods of less than a year controlling average algal biomass (Biggs 1996b). Clausen and Biggs (1998) stated that the absolute magnitude of the flow and some measure of flow variability were significantly related to a number of biological variables. For example, they found that when bed stability, conductivity, and flow frequency measures were incorporated into a chlorophyll-nutrient model, up to 70% of the variability in chlorophyll was explained (Clausen and Biggs 1998). While our study focused on sampling during the relatively stable summer flow periods, the Base Flow Index (BFI) (Wolock 2003a) was used as an indicator of the fraction of annual streamflow that occurs during baseflow. A stream with a high BFI value derives a large proportion of its annual flow from groundwater (e.g. more stable flow), whereas a stream with a low BFI is influenced primarily by surface runoff (e.g. more flashy flows). The BFI was a significant factor in two CHLFG models (ALL and GCP) and two CHLS models (GCP and CCYK). In all four cases, the BFI had a negative association with algal biomass, indicating that streams with a greater portion of their flow derived from groundwater tended to have lower algal biomass. The finding that algal biomass was greater in streams dominated by surface water runoff (more flashy) may seen counter intuitive to studies that have shown the negative influence of flow fluctuations on algal biomass. However, the BFI in this study was based upon an annual period and does not reflect short-term changes in flow prior to sampling. When all sites were combined there was a significant negative correlation between BFI and both TN (r = -0.32) and TP (r = -0.27), although by study area the relationship varied. The higher algal biomass in surface water dominated streams may reflect the transport of nutrients, particularly TP, into streams over the course of a year.
Along with temperature and flow, light is the third key variable commonly cited as having substantial influence on algal communities (Chetelat and others 1999). In this study, percent canopy cover was used to indirectly assess the influence of light on algal biomass. Canopy was an important variable in the ALL regression models, as well as the DLMV CHLFG model. Riparian shading is well documented to be an important factor in controlling algal biomass. Streams with relatively open canopy cover generally contain higher algal biomass than streams with more cover (Lowe and others 1986; Quinn and others 1997; Mosisch and others 2001). In our study, open canopy streams (CAN <50%) contained the greatest biomass, with biomass negatively correlated with nutrient concentrations; closed canopy systems (CAN >50%) showed the opposite effect.
Nutrient-Algal Biomass Conceptual Model
The Nutrient-Algal Biomass Conceptual Model (Fig. 3) provides a framework for understanding why study areas or sites deviate from predicted norms. The solid line in Fig. 1 represents a linear response of algal biomass as a function of increasing nutrient concentration. The lower dashed line represents a threshold at which algal biomass begins to rapidly increase (Stevenson and others 2006), while the upper dashed line represents potential nutrient saturation. The development of nutrient criteria partially depends on models that can accurately predict some biological response (e.g. biomass) as a function of nutrient concentrations; however, a variety of processes can greatly influence this relationship. For example, the upper left quadrant of Fig. 3 includes sites where algal biomass is sufficiently elevated to reduce nutrient concentrations through biological uptake, whereas the lower right quadrant of Fig. 3 includes sites where habitat (e.g., lack of light penetration, scouring) or biological factors (e.g., grazing) controls algal growth. Because sites in the lower right quadrant have high concentrations of nutrients and low processing they are major nutrient contributors to downstream waters.
Nutrient-Algal Biomass Conceptual Model illustrating the interaction of nutrients and algal biomass (chlorophyll a). The solid line represents a linear response of algal biomass as a function of increasing nutrient concentration. Individual sites fall into one of the four quadrants depending on nutrient-biomass interactions
Our study demonstrates that the interpretation of nutrient and algal biomass data is partly dependent on the scale of the assessment, and that combining data from large-scale studies may mask smaller scale interactions. For example, algal biomass increased as a function of increased TP when all study areas were combined; however, different patterns were found at smaller spatial scales. Nutrient and chlorophyll concentrations at most of the sites in the GCP fell in the lower left quadrant of Nutrient-Algal Biomass Model (Fig. 3), indicating that most are below any threshold value or are just within the rapid biomass accumulation phase. The DLMV was the only study area that demonstrated the commonly predicted nutrient-biomass relationship with a sufficient gradient from low to high nutrients (Fig. 2e). The CCYK data (Fig. 2c) showed the opposite of the predicted pattern; higher TP concentrations were associated with lower chlorophyll values. Therefore, the CCYK included sites where algal biomass may control nutrient concentrations (upper left quadrant of Fig. 3) and sites where habitat is limiting and nutrients are therefore transported downstream (lower right quadrant of Fig. 3). In contrast, the CNBR and WHMI had all or most of their sites with high nutrient and algal biomass (upper right quadrant of Fig. 3) indicating potential nutrient saturation. Additional algal growth at these sites may be limited by physical (e.g. light limitation) or biological (e.g. grazing) factors (Fig. 2, d and f) but not to a degree that severely depletes the algal biomass.
Implications for Nutrient Criteria and Management
The goal of setting regional nutrient criteria is to improve and/or protect the ecological health of local and downstream waters. Much of this effort has focused on setting regional level TN and TP criteria, with some effort on assessing the relations between nutrients and algal biomass. Nutrient loading to agricultural streams in some regions may be high enough that nutrient concentrations could greatly exceed what algae and aquatic macrophytes require for growth, therefore reductions in nutrient loads may need to be substantial. The role of stream and riparian habitat in agricultural streams determines nutrient-biota relations and in many agricultural streams habitat can be limiting to aquatic biota. Complex and inconsistent interactions of nutrients and algal biomass in agricultural streams underscores the need to include additional biological indicators of nutrient enrichment, such as algal, invertebrate, or fish assemblage indicators.
Regardless of the strategy that is adopted for managing nutrients in agricultural streams, it is important to have sufficient knowledge about a stream system in order to set realistic expectations as to improvements in biological conditions as a result of management practices. For example, along with high nutrient loading, many agricultural streams have reduced retention time due to the alteration of stream channels. This can result in a low percentage of nutrient removal (Duff and others 2008). Furthermore, the potential legacy effects from groundwater sources of nitrate or OP can have long lag times between nutrient applications to the land surface and discharge to streams (Tesoriero and others 2009). Although the reduction of nutrient loads may not always result in rapid decreases in nutrient concentrations or improvements in biological condition in the local streams, downstream waters will benefit because of the large number of small streams that are sources of nutrients to downstream ecosystems.
Conclusions
This study is one of the first to compare nutrients, algal biomass, and habitat among five major agricultural regions in the United States. The use of TN and TP concentrations alone indicated a relatively high percentage of nutrient enriched sites; whereas, algal biomass, expressed as chlorophyll a, varied due to site-specific processes. Although large-scale national regression models can be developed, regional nutrient-algal interactions vary greatly. This study demonstrated the importance of including stream and riparian habitat features in models that predict algal biomass as a function of nutrient concentrations, with canopy, temperature, substrate, and flow determined to be important. This study also presents a Nutrient-Algal Biomass Conceptual Model that helps explain why sites and/or areas may not show the expected relation between nutrients and algal biomass.
References
Arar EJ, Collins GB (1997) U.S. Environmental Protection Agency Method 445.0. In vitro determination of chlorophyll a and pheophytin a in marine and freshwater algae by fluorescence, Revision 1.2. U.S. Environmental Protection Agency, National Exposure Research Laboratory. Office of Research and Development, Cincinnati, OH
Biggs BJF (1995) The contribution of flood disturbance, catchment geology, and landuse to the habitat template of periphyton in stream ecosystems. Freshwater Biology 33:419–438
Biggs BJF (1996a) Patterns in benthic algae in streams. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, CA, pp 31–76
Biggs BJF (1996b) Hydraulic habitat of plants in streams. Regulated Rivers: Research and Management 12:131–144
Biggs BJF, Close ME (1989) Periphyton biomass dynamics in gravel bed rivers: the relative effects of flows and nutrients. Freshwater Biology 22:209–231
Biggs BJF, Shand BI (1987) Biological communities and the potential effects of power development in the lower Clutha River, Otago. Ministry of Works and Development, Christchurch, NZ
Bowes MJ, Smith JT, Hilton J, Sturt MM, Armitage PD (2007) Periphyton biomass response to changing phosphorous concentrations in a nutrient-impacted river: a new methodology for phosphorous target settings. Canadian Journal of Fisheries and Aquatic Sciences 64:227–238
Brezonik RL, Easter KW, Hatch L, Mulla D, Perry J (1999) Management of diffuse pollution in agricultural watersheds: Lessons learned from the Minnesota River Basin. Water Science and Technology 39:323–330
Carpenter SR, Caraco NF, Correl DL, Howarth RW, Sharpley N, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8:559–568
Chetelat J, Pick FR, Morin A, Hamilton PB (1999) Periphyton biomass and community composition in rivers of different nutrient status. Canadian Journal of Fisheries and Aquatic Sciences 56:560–569
Clausen B, Biggs BJF (1998) Streamflow variability indices for riverine environmental studies. Hydrology in a changing environment. In: Proceedings of the British Hydrological Society International Conference, pp 357–364
Correll DL (1998) The role of phosphorus in the eutrophication of receiving waters: a review. Journal of Environmental Quality 27:261–266
Dodds WK, Smith VH, Lohman K (2002) Nitrogen and phosphorus relationships to benthic algal biomass in temperature streams. Canadian Journal of Fisheries and Aquatic Sciences 59:865–874
Duff JH, Tesoriero AJ, Richardson WB, Strauss EA, Munn MD (2008) Whole-stream response to nitrate loading in three streams draining agricultural landscapes. Journal of Environmental Quality 37:1133–1144
Fishman MJ (1993) Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of inorganic and organic constituents in water and fluvial sediments. U.S. Geological Survey Open-File Report 93–125, Denver, CO
Fitzpatrick FA, Waite IR, D’Arconte PJ, Meador MR, Maupin MA, Gurtz ME (1998) Revised methods for characterizing stream habitat in the National Water-Quality Assessment Program. Water-Resource Investigation Report 98-4052
Fuhrer GJ, Gilliom RJ, Hamilton PA, Morace J, Nowell LH, Rinella JR, Stoner JD, Wentz DA (1999) The quality of our nation’s waters: nutrients and pesticides. U.S. Geological Survey Circular 1225
Johnson MR, Zelt RB (2005) Protocols for mapping and characterizing land use/land cover in Riparian Zones. U.S. Geological Survey Open-File Report 2005–1302
Jowett IG, Biggs BJF (1997) Flood and velocity effects on periphyton and silt accumulation in two New Zealand rivers. New Zealand Journal of Marine and Freshwater Research 31:287–300
Kilkus SP, LaPerriere JD, Backmann RW (1975) Nutrients and algae in some central Iowa streams. Journal of Water Pollution Control Federation 47:1870–1879
Kjeldsen K (1994) The relationship between phosphorus and peak biomass of benthic algae in small lowland streams. Verhandlungen der Internationale Vereinigung für Theoretische und Angewandte Limnologie 25:1530–1533
Kosinski RJ (1984) The effect of terrestrial herbicides on the community structure of stream periphyton. Environmental Pollution 36:165–189
Lamberti GA, Resh VH (1983) Stream periphyton and insect herbivores: an experimental study of grazing by a caddisfly population. Ecology 64:1124–1135
Lohman K, Jones JR, Perkins BD (1992) Effects of nutrient enrichment and flood frequency on periphyton biomass in northern Ozark streams. Canadian Journal of Fisheries and Aquatic Sciences 49:1198–1205
Lowe RL, Golladay SW, Webster JR (1986) Periphyton response to nutrient manipulation in streams draining clearcut and forested watersheds. Journal of the North American Benthological Society 5:221–229
Mosisch TD, Bunn SE, Davis PM (2001) The relative importance of shading and nutrients on algal production in subtropical streams. Freshwater Biology 46:1269–1278
Moulton SR, Kennen JG, Goldstein RM, Hambrook JA (2002) Revised protocols for sampling algal, invertebrate, and fish communities as part of the National Water-Quality Assessment Program. U.S. Geological Survey Open-File Report 02-150
Mueller DK, Spahr NE (2006) Nutrients in streams and rivers across the Nation—1992–2001. U.S. Geological Survey Scientific Investigations Report 2006–5107
Munn MD, Osborne LL, Wiley MJ (1989) Factors influencing periphyton growth in agricultural streams of central Illinois. Hydrobiologia 174:89–97
Nakagaki N, Wolock DM (2005) Estimation of agricultural pesticide use in drainage basins using land cover maps and county pesticide data. U.S. Geological Survey Open-File Report 2005-1188
NRC (National Research Council) (2000) Clean coastal waters: understanding and reducing the effects of nutrient pollution. National Academy Press, Washington, DC
Patton CJ, Kryskalla JR (2003) Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory—evaluation of alkaline persulfate digestion as an alternative to kjeldahl digestion for determination of total and dissolved nitrogen and phosphorus in water. U.S. Geological Survey Open-File Report 03-4174
Patton CJ, Truitt EP (2000) Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory–Determination of ammonium plus organic nitrogen by a Kjeldahl digestion method and an automated photometric finish that includes digest cleanup by gas diffusion. U.S. Geological Survey Open-File Report 00-170
Powers ME (1992) Hydrologic and trophic controls of seasonal algal blooms in northern California rivers. Archives Hydrobiologia 125:385–410
Quinn JM, Copper AB, Stroud MJ, Burrell GP (1997) Shade effects on stream periphyton and invertebrates: an experiment in streamside channels. New Zealand Journal of Marine and Freshwater Research 31:665–683
Rabalais NN, Turner RE, Dortch Q, Wiseman WJ, Gupta BKS (1996) Nutrient changes in the Mississippi River and system responses on the adjacent continental shelf. Estuaries 19:386–407
Riseng CM, Wiley MJ, Stevenson RJ (2004) Hydrologic disturbance and nutrient effects on benthic community structure in midwestern U.S. streams: a covariance structure analysis. Journal of the North American Benthological Society 23:309–326
Ruddy BC, Lorenz DL, Mueller DK (2006) County-level estimates of nutrient inputs to the land surface of the conterminous United States, 1982–2001
Sand-Jensen (1998) Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshwater Biology 39:663–679
Shelton LR (1994) Field guide for collecting and processing stream-water samples for the National Water-Quality Assessment Program. U.S. Geological Survey Open-File Report 94-455. U.S. Geological Survey, Reston, VA
Smith VH, Tilman GD, Nekola JC (1999) Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100:179–196
Stevenson RJ, Stoermer EF (1981) Quantitative differences between benthic algal communities along a depth gradient in Lake Michigan. Journal of Phycology 17:29–36
Stevenson RJ, Rier ST, Riseng CM, Schultz RE, Wiley MJ (2006) Comparing effects of nutrients on algal biomass in streams in two regions with different disturbance regimes and with applications for developing nutrient criteria. Hydrobiologia 561:149–165
Tesoriero AJ, Duff JH, Wolock DM, Spahr NE, Almendinger JE (2009) Identifying pathways and processes affecting nitrate and orthophosphate inputs to streams in agricultural watersheds. Journal of Environmental Quality 38:1892–1900
Turner RE, Rabalais NN (1994) Coastal eutrophication near the Mississippi River delta. Nature 368:619–621
U.S. EPA (1998) National strategy for the development of regional nutrient criteria. U.S. Environmental Protection Agency Office of Water Fact Sheet EPA-822-F-98-002. http://www.epa.gov/waterscience/criteria/nutrient/nutsi.html. Accessed November 2006
U.S. EPA (2000) National Water Quality Inventory: 2000 Report to Congress. Office of Water EPA-841-R-02-001
U.S. EPA (2002) National Water Quality Inventory—2000 Report. Washington, D.C., Office of Water, Report No. EPA-841-R-02-01. http://www.epa.gov/305b/2000report/. Accessed 30 April 2007
U.S. EPA (2006) Wadeable Streams Assessment. Office of Water EPA 841-B-06-002: Washington, DC
U.S. Geological Survey and U.S. Environmental Protection Agency (2003) National Hydrography Dataset (NHD) [digital data]. http://www.nhd.usgs.gov. Accessed 15 June 2003
van Nieuwenhuyse EE, Jones JR (1996) Phosphorous-chlorophyll relationship in temperate streams and its variation with stream catchment area. Canadian Journal of Fisheries and Aquatic Sciences 53:99–105
Vogelmann JE, Howard SM, Yang L, Larson CR, Wyie BK, Van Driel N (2001) Completion of the 1990’s national land cover dataset for the conterminous United States from Landsat Thematic Mapper data and ancillary data sources. Photogrammetric Engineering and Remote Sensing 67:650–662
Wahl K L, Wahl TL (1995) Determining the flow of Comal Springs at New Braunfels, Texas, Texas Water ‘95, American Society of Civil Engineers Symposium, San Antonio, Texas, August 16–17, 1995
Welch EB (1992) Ecological effects of wastewater. Chapman and Hall, London
Welch FB, Jacoby JM, Horner RI, Seeley MR (1988) Nuisance biomass levels of periphytic algae in streams. Hydrobiologia 157:161–168
Wharton G, Cotton JA, Wotton RS, Bass JAB, Heppell CM, Trimmer M, Sanders IA, Warren LL (2006) Macrophytes and suspension-feeding invertebrates modify flows and fine sediments in the Frome and Piddle catchments, Dorset (UK). Journal of Hydrology 330:171–184
Wolock DM, (2003a) Base-flow index grid for the conterminous United States, U.S. Geological Open-File Report 03-263, Metadata report and data available on the World Wide Web at URL http://www.water.usgs.gov/lookup/getspatial?bfi48grd
Wolock DM (2003b) Flow characteristics at U.S. Geological Survey streamgages in the conterminous United States, U.S. Geological Survey Open-File Report 03-146, Metadata report and data available on the World Wide Web at URL http://water.usgs.gov/lookup/getspatial?qsitesdd
Acknowledgments
This study could not have been done without the data collection efforts of Robert Black, Daniel Calhoun, Brian Caskey, Jill Frankforter, Brian Gregory, Patrick Moran, and Holly Weyers. The authors would like to also thank Jerad Bales and Barbara Scudder for technical review, along with journal reviewers. This study was funded as part of the U.S. Geological Survey’s National Water Quality Assessment Program, Nutrient Effects on Stream Ecosystems (http://www.wa.water.usgs.gov/neet/).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Munn, M., Frey, J. & Tesoriero, A. The Influence of Nutrients and Physical Habitat in Regulating Algal Biomass in Agricultural Streams. Environmental Management 45, 603–615 (2010). https://doi.org/10.1007/s00267-010-9435-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-010-9435-0